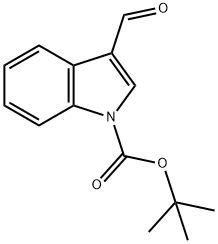
TERT-BUTYL 3-FORMYL-1H-INDOLE-1-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 3-FORMYL-1H-INDOLE-1-CARBOXYLATE
- CAS Number:57476-50-3
- Molecular formula:C14H15NO3
- Molecular Weight:245.27
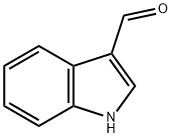
487-89-8

24424-99-5

57476-50-3
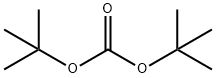
GENERAL METHOD: 3-indolecarboxaldehyde, triethylamine (1.3 eq.) and 10 mol% DMAP were dissolved in dichloromethane and di-tert-butyl dicarbonate (1.2 eq.) was added slowly. The reaction mixture was stirred at room temperature until complete consumption of the ingredients was shown by thin layer chromatography (TLC) monitoring. Upon completion of the reaction, the reaction mixture was diluted with dichloromethane and subsequently washed with water. The organic layer was separated, washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by fast column chromatography (eluent ratio: ethyl acetate/hexane = 1:5 for 1a and 1b; ethyl acetate/hexane = 1:9 for 1c) to afford the target compounds tert-butyl 3-formyl-1H-indole-1-carboxylate (1a-1c).
5470-70-2
536 suppliers
$5.00/5g

24424-99-5
868 suppliers
$13.50/25G

57476-50-3
120 suppliers
$6.00/1g
Yield: 8.04 g
Reaction Conditions:
with dmap;triethylamine in dichloromethane at 20; for 1 h;
Steps:
23 Synthesis of (5-bromo-2-pyridyl)-(1-methylindol-3-yl)methanone (Intermediate 33)
Method 23
Synthesis of (5-bromo-2-pyridyl)-(1-methylindol-3-yl)methanone (Intermediate 33)
To a solution of R-8 (5.00 g, 34.45 mmol), DMAP (0.42 g, 3.44 mmol) and triethylamine (9.6 mL, 68.89 mmol) in DCM (300 mL) is added di-tert-butyl dicarbonate (8.27 g, 37.89 mmol) and the resultant solution stirred at room temperature for 1 h.
The solution is washed with saturated aqueous NH4Cl and brine then dried over anhydrous Na2SO4.
The solvent is removed in vacuo to give I-29 (8.04 g).
References:
BARTOLOZZI, Alessandra;CHEN, Zhidong;DINES, Jonathon Alan;LO, Ho Yin;LOKE, Pui Leng;OLAGUE, Alan;RIETHER, Doris;TYE, Heather;WU, Lifen;ZINDELL, Renee M. US2013/196967, 2013, A1 Location in patent:Paragraph 0224; 0225

487-89-8
626 suppliers
$6.00/10g

24424-99-5
868 suppliers
$13.50/25G

57476-50-3
120 suppliers
$6.00/1g
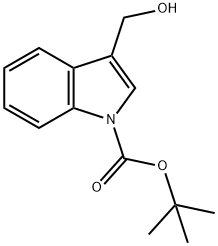
96551-22-3
101 suppliers
$10.00/250mg

57476-50-3
120 suppliers
$6.00/1g
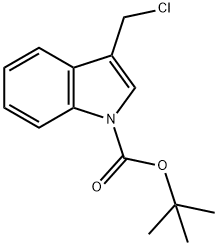
862704-32-3
6 suppliers
$1320.00/1g

57476-50-3
120 suppliers
$6.00/1g