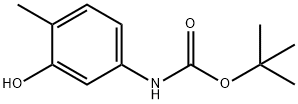
tert-butyl 3-hydroxy-4-methylphenylcarbamate synthesis
- Product Name:tert-butyl 3-hydroxy-4-methylphenylcarbamate
- CAS Number:345893-26-7
- Molecular formula:C12H17NO3
- Molecular Weight:223.27

24424-99-5
871 suppliers
$13.50/25G
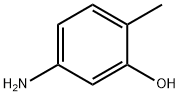
2835-95-2
423 suppliers
$8.00/25g

345893-26-7
35 suppliers
$39.00/100mg
Yield:345893-26-7 18%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 0 - 20; for 15 h;
Steps:
32
Reference Example 32; tert-butyl (3-hydroxy-4-methylphenyl) carbamate; To a solution of 5-amino-2-methylphenol (10.0 g, 81.2 mmol) and triethylamine (16.9 rtiL, 122 mmol) in tetrahydrofuran (75 mL) was added dropwise with stirring under ice-cooling a solution of di-tert-butyl-dicarbonate (19.5 g, 89.3 mmol) in tetrahydrofuran(25 mL) , and the mixture was stirred at room temperature for 15 hr. The reaction mixture was diluted with water and extracted with ethyl acetate. The organic layer was washed with water and saturated brine, dried over anhydrous magnesium sulfate and filtrated. The filtrate was concentrated under reduced pressure, and the residue was purified by column chromatography (silica gel, hexane/ethyl acetate =95/5->50/50) to give the title compound (3.25 g, 18%) as a colorless oil.1H-NMR (DMSOd6, 300 MHz) δ 1.46 (9H, s) , 2.02 (3H, s) , 6.71 (IH, dd, J = 8.2, 1.8 Hz), 6.87 (IH, d, J = 8.2 Hz), 7.07 (IH, d, J = 1.8 Hz), 9.09 (IH, s) , 9.16 (IH, s) .
References:
WO2008/150015,2008,A1 Location in patent:Page/Page column 182