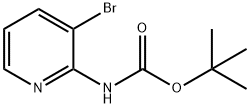
CARBAMIC ACID, (3-BROMO-2-PYRIDINYL)-, 1,1-DIMETHYLETHYL ESTER synthesis
- Product Name:CARBAMIC ACID, (3-BROMO-2-PYRIDINYL)-, 1,1-DIMETHYLETHYL ESTER
- CAS Number:149489-04-3
- Molecular formula:C10H13BrN2O2
- Molecular Weight:273.13
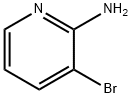
13534-99-1
360 suppliers
$4.00/1g

24424-99-5
871 suppliers
$13.50/25G

149489-04-3
35 suppliers
$117.00/50mg
Yield:149489-04-3 63%
Reaction Conditions:
Stage #1: 2-Amino-3-bromopyridine;di-tert-butyl dicarbonatewith dmap;triethylamine in dichloromethane at 20; for 2.5 h;
Stage #2: with methanol;potassium carbonate at 65; for 5 h;
Steps:
A1 Alternative preparation of intermediate 2:
A solution of 2-amino-3-bromopyridine (100 g, 578 mmol) in DCM (700 mL) wastreated with TEA (170 mL, 1219.7 mmol) and DMAP (3.50 g, 5.73 mmol) and asolution of Boc2O (265 g, 1214.22 mmol) in DCM (100 mL) was added over 30 mm.The solution was stirred at room temp for 2 h. The completion of the reaction wasdetermined by TLC (EtOAc 30%, cyclohexane 70%). The mixture was evaporatedunder vacuum and the residue was dissolved in MeOH (1000 mL), treated with K2C03(200 g, 1447.12 mmol) and heated to 65 °C for 5 h. The reaction mixture was filteredand the filtrate was evaporated under vacuum. The residue was partitioned betweenethyl dichloro methane and water and the organic layer was washed with water, 1 Mcitric acid solution, brine, dried over Na2SO4 and filtered. The filtrate was stirred withflash silica gel, filtered and evaporated under vacuum. The residue was triturated withpetroleum ether 40 - 60°C to give 99.7 g of intermediate 2 (63% yield, pinkish solid).
References:
WO2019/8011,2019,A1 Location in patent:Page/Page column 110; 111
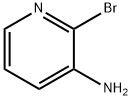
39856-58-1
307 suppliers
$10.00/1g

24424-99-5
871 suppliers
$13.50/25G

149489-04-3
35 suppliers
$117.00/50mg
29786-93-4
0 suppliers
inquiry
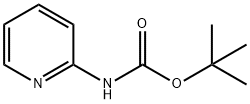
38427-94-0
139 suppliers
$10.00/1g

106-93-4
405 suppliers
$15.00/5g

149489-04-3
35 suppliers
$117.00/50mg

13534-99-1
360 suppliers
$4.00/1g

149489-04-3
35 suppliers
$117.00/50mg
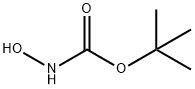
36016-38-3
361 suppliers
$6.00/5g

149489-04-3
35 suppliers
$117.00/50mg
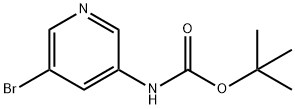
361550-43-8
119 suppliers
$9.00/250mg