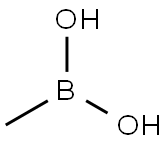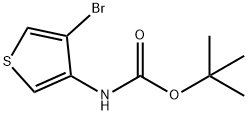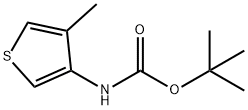
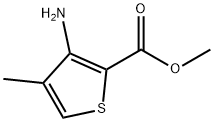
tert-butyl 4-methylthiophen-3-ylcarbamate synthesis
- Product Name:tert-butyl 4-methylthiophen-3-ylcarbamate
- CAS Number:1097629-78-1
- Molecular formula:C10H15NO2S
- Molecular Weight:213.3

24424-99-5
814 suppliers
$13.50/25G
85006-31-1
349 suppliers
$5.00/5g

1097629-78-1
7 suppliers
inquiry
Yield:1097629-78-1 100%
Reaction Conditions:
Stage #1: 3-amino-4-methyl-2-thiophenecarboxylic acid methyl esterwith potassium hydroxide in water at 20 - 80; for 0.5 h;
Stage #2: with hydrogenchloride in water at 50 - 60; for 0.25 h;
Stage #3: di-tert-butyl dicarbonatewith potassium hydroxide in hexane;water;acetone at -10 - 20;
Steps:
1 Step 1:
tert-butyl (4-methylthiophen-3-yl)carbamate:
To a solution of methyl 3-amino-4-methylthiophene-2-carboxylate (1.71 g, 10.0 mmol) in H2O (5.1 mL) was added KOH solution (45 %, 1.90 mL, 14.0 mmol, 1.4 eq.) at room temperature.
The reaction was stirred at 80 °C for 30 min and afterwards cooled to room temperature.
This solution was slowly added to a 6 M HCl solution (5.50 mL, 33.0 mmol, 3.3 eq) at 50 °C.
After completion of the addition, the reaction was stirred at 60 °C for 15 min (gas evolution, clear solution was obtained).
The solution was cooled to 5 °C (ice bath), hexanes (3.5 mL) was added, and the solution cooled to -10 °C (acetone, dry ice).
A solution of KOH (45 %, 3.70 mL, 27.0 mmol, 2.7 eq.) and di-tert-butyl dicarbonate (2.40 mL, 10.5 mmol, 1.05 eq.) were added at -10 °C.
The reaction solution was stirred overnight and slowly warmed to room temperature.
The formed suspension was extracted with EtOAc (3*15 mL).
The combined organic layers were washed with a saturated aqueous solution of NaHCO3 (1*25 mL), dried over Na2SO4, concentrated and filtered. tert-butyl (4-methylthiophen-3-yl)carbamate (2.32 g, quantitative yield) was obtained as an orange solid and used without further purification.
Rf = 0.68 (cHex/EtOAc 9:1, UV 254 nm).
1H NMR (CDCl3, 400 MHz): δ 7.40 (s, 1H), 6.86 (dq, J = 3.3 Hz, J = 1.1 Hz, 1H), 6.36 (s, 1H), 2.14 (d, J = 1.1 Hz, 3H), 1.53 (s, 9H).
References:
EP3643713,2020,A1 Location in patent:Paragraph 0466-0468

24424-99-5
814 suppliers
$13.50/25G
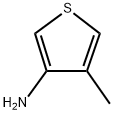
23967-97-7
54 suppliers
$53.00/100mg

1097629-78-1
7 suppliers
inquiry
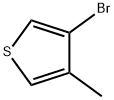
30318-99-1
217 suppliers
$6.00/1g

1097629-78-1
7 suppliers
inquiry