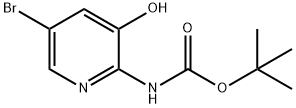
tert-butyl 5-bromo-3-hydroxypyridin-2-ylcarbamate synthesis
- Product Name:tert-butyl 5-bromo-3-hydroxypyridin-2-ylcarbamate
- CAS Number:1207175-73-2
- Molecular formula:C10H13BrN2O3
- Molecular Weight:289.13

24424-99-5
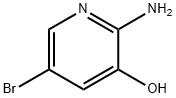
39903-01-0

1207175-73-2
Example 1 Synthesis of 5-bromo-3-hydroxy-2-tert-butoxycarbonylaminopyridine (0038): 2-amino-3-hydroxy-5-bromopyridine (10.0 g, 53.0 mmol) and triethylamine (10 mL, 71.8 mmol) were added to dichloromethane (100 mL) followed by di-tert-butyl dicarbonate (12.7 g, 58.4 mmol). The reaction mixture was stirred at room temperature for 18 hours. Water (150 mL) was added and stirring was continued for 30 minutes. The reaction mixture was filtered through diatomaceous earth to separate the organic layer. The aqueous layer was extracted with dichloromethane (150 mL). The combined organic layers were washed with saturated aqueous sodium chloride (2 x 100 mL) and dried over anhydrous sodium sulfate. The solvent was removed by concentration under reduced pressure and the residue was ground with hexane (100 mL), filtered and dried in vacuum to give 5-bromo-3-hydroxy-2-tert-butoxycarbonylaminopyridine (15.0 g, 98.0% yield) as a white solid. Product characterization data: 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 2.0 Hz, 1H), 7.58 (d, J = 2.0 Hz, 1H), 4.67 (br s, 2H), 1.56 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 150.4, 150.1, 145.3, 133.5, 131.3 133.5, 131.5, 106.5, 85.0, 27.6.

24424-99-5
861 suppliers
$13.50/25G

39903-01-0
217 suppliers
$15.00/1g

1207175-73-2
79 suppliers
$13.00/100mg
Yield: 98%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 18 h;
Steps:
1 Example 1 The preparation of 5-bromo-3-hydroxy-2-tert-butyloxycarbonylamino pyridine
Example 1 The preparation of 5-bromo-3-hydroxy-2-tert-butyloxycarbonylamino pyridine (0038) To a solution of 2-amino-3-hydroxy-5-bromopyridine (10.0 g, 53.0 mmol) and Et3N (10 mL, 71.8 mmol) in dichloromethane (100 mL) was added Boc2O (12.7 g, 58.4 mmol). The mixture was stirred at room temperature for 18 h and continued to stir for 30 min after the addition of 150 ml water. The reaction mixture was filtrated off through celite. The organic layer was separated and the aqueous layer was extracted with 150 dichloromethane. The combined organic layers were washed with saturated NaCl aqueous solution (2×100 mL) and then dried over anhydrous Na2SO4. The solvent was removed under reduced pressure and the obtained residue was triturated in hexane (100 mL), filtrated, and dried under vacuum to afford 15.0 g 5-bromo-3-hydroxy-2-tert-butyloxycarbonylamino pyridine as a white solid, with a yield of 98.0%. (0039) 1H NMR (400 MHz, CDCl3): δ 7.98 (d, J=2.0 Hz, 1H), 7.58 (d, J=2.0 Hz, 1H), 4.67 (brs, 2H), 1.56 (s, 9H); 1C NMR (100 MHz, CDCl3): δ 150.4, 150.1, 145.3, 133.5, 131.5, 106.5, 85.0, 27.6.
References:
ZHEJIANG JIUZHOU PHARMACEUTICAL CO., LTD.;LI, Yuanqiang;QIAN, Jianqiang;CHE, Daqing US2015/307476, 2015, A1 Location in patent:Paragraph 0038-0039
16867-03-1
588 suppliers
$10.00/25g

1207175-73-2
79 suppliers
$13.00/100mg
![2,3-Dihydropyrido[2,3-d][1,3]oxazol-2-one](/CAS/GIF/60832-72-6.gif)
60832-72-6
206 suppliers
$5.00/1g

1207175-73-2
79 suppliers
$13.00/100mg
![6-BROMO-3H-OXAZOLO[4,5-B]PYRIDIN-2-ONE](/CAS/GIF/21594-52-5.gif)
21594-52-5
136 suppliers
$10.00/250mg

1207175-73-2
79 suppliers
$13.00/100mg