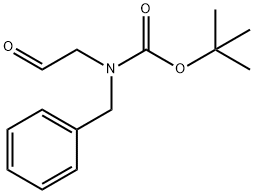
tert-butyl benzyl(2-oxoethyl)carbamate synthesis
- Product Name:tert-butyl benzyl(2-oxoethyl)carbamate
- CAS Number:136159-63-2
- Molecular formula:C14H19NO3
- Molecular Weight:249.31

24424-99-5
868 suppliers
$13.50/25G
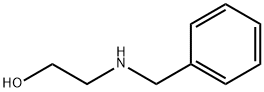
104-63-2
360 suppliers
$7.00/10g

136159-63-2
5 suppliers
inquiry
Yield: 86%
Reaction Conditions:
Stage #1:di-tert-butyl dicarbonate;N-Benzylethanolamine in dichloromethane at 20; for 1 h;
Stage #2: with Dess-Martin periodane in dichloromethane at 20; for 0.5 h;
Steps:
15A
Boc2O (7.68 mL, 33.1 mmol) was added to a cooled solution of 2- (benzylamino)ethanol (5.00 g, 33.1 mmol) in DCM (132 mL), and the reaction mixture was warmed to rt and stirred for 1h. Dess-Martin Periodinane (DMP) (14.0 g, 33.1 mmol) was added to the reaction mixture and stirring at rt was continued for an additional 30 min. The reaction mixture was quenched with a 1:1 mixture of sat. aq. NaHCO3: 10% aqueous sodium thiosulfate. The mixture was concentrated, and product isolated by silica gel chromatography to provide 15A as a clear, colorless oil. (7.10 g, 86 %).1H NMR (500MHz, CDCl3) δ 9.64 - 9.35 (m, 1H), 7.52 - 7.13 (m, 5H), 4.66 - 4.43 (m, 2H), 4.05 - 3.70 (m, 2H), 1.51 (br d, J=13.2 Hz, 9H)
References:
BRISTOL-MYERS SQUIBB COMPANY;SMALLHEER, Joanne M.;HU, Carol Hui;VALENTE, Meriah Neissel;SHAW, Scott A.;VOKITS, Benjamin P.;HALPERN, Oz Scott WO2017/160632, 2017, A1 Location in patent:Page/Page column 89; 90
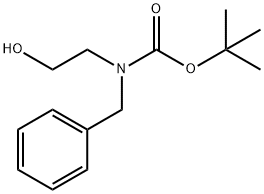
121496-39-7
33 suppliers
$36.00/100mg

136159-63-2
5 suppliers
inquiry

7732-18-5
507 suppliers
$12.69/100ml

121496-39-7
33 suppliers
$36.00/100mg

136159-63-2
5 suppliers
inquiry

104-63-2
360 suppliers
$7.00/10g

136159-63-2
5 suppliers
inquiry
6436-90-4
375 suppliers
$8.00/10g

136159-63-2
5 suppliers
inquiry