
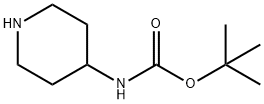
tert-butyl N-(1-cyanopiperidin-4-yl)carbamate synthesis
- Product Name:tert-butyl N-(1-cyanopiperidin-4-yl)carbamate
- CAS Number:721450-38-0
- Molecular formula:C11H19N3O2
- Molecular Weight:225.29
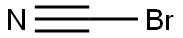
506-68-3
166 suppliers
$10.00/1g
73874-95-0
440 suppliers
$10.00/250mg

721450-38-0
9 suppliers
inquiry
Yield:-
Reaction Conditions:
with sodium hydrogencarbonate in dichloromethane at 0 - 20; for 3 h;
Steps:
72
To a solution of piperidin-4-ylcarbamic acid tert-butyl ester (10. Og, 49.9mmol) in DCM (130mL) was added a slurry of NaHC03 (12.6g, 149.8mmol) in water (30mL) and the resulting mixture was cooled to 0°C. A solution of cyanogen bromide (6.4g, 59.9mmol) in DCM (15mL) was added, dropwise, over 10 min then the mixture was warmed to r.t and stirred for 3 h. The reaction mixture was diluted with water, then the layers were separated. The aqueous phase was extracted with DCM, then the organic fractions were combined, washed with sat. NaHC03 solution, then brine, and dried (MgS04). Removal of the solvent in vacuo afforded the title compound: RT = 2.93 min; mlz (ES ) = 226.1 [M+ H]+.
References:
PROSIDION LIMITED;BARBA, Oscar;BELL, James, Charles;DUPREE, Tom, Banksia;FRY, Peter, Timothy;BERTRAM, Lisa, Sarah;FYFE, Matthew, Colin, Thor;GATTRELL, William;JEEVARATNAM, Revathy, Perpetua;KEILY, John;KRULLE, Thomas, Martin;MCDONALD, Russell, Walker;MORGAN, Trevor;RASAMISON, Chrystelle, Marie;SCHOFIELD, Karen, Lesley;STEWART, Alan, John, William;SWAIN, Simon, Andrew;WITHALL, David, Matthew WO2011/147951, 2011, A1 Location in patent:Page/Page column 57