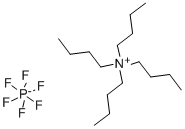
Tetrabutylammonium hexafluorophosphate synthesis
- Product Name:Tetrabutylammonium hexafluorophosphate
- CAS Number:3109-63-5
- Molecular formula:C16H36F6NP
- Molecular Weight:387.43
1643-19-2

3109-63-5
The general procedure for the synthesis of tetrabutylammonium hexafluorophosphate from tetrabutylammonium bromide was as follows: potassium hexafluorophosphate (K[PF6], 2.055 g, 11.2 mmol) was dissolved in 30 mL of deionized water. Tetrabutylammonium bromide ([(n-Bu4)N]Br, 3.601 g, 11.2 mmol) was dissolved in 30 mL of dichloromethane (CH2Cl2) and the resulting solution was subsequently added to the aqueous potassium hexafluorophosphate solution. After stirring the reaction mixture for 24 h at room temperature, phase separation was carried out. The organic phase was washed three times with 10 mL of deionized water, followed by drying with anhydrous sodium carbonate (Na2CO3) and filtration. The filtrate was concentrated by rotary evaporation to give a white solid product. The resulting solid was dried under vacuum at 80 °C for 18 h to give 3.822 g of tetrabutylammonium hexafluorophosphate ([(n-Bu4)N][PF6]) in 88% yield (9.87 mmol). The product characterization data are as follows: differential scanning calorimetry (DSC, 10 K/min): melting point (m.p.) = 250 °C, decomposition temperature (Tdec) = 388 °C. Calculated elemental analysis (measured values): C 49.60% (49.97%), H 9.37% (9.00%), N 3.61% (3.61%).1H NMR (25 °C, CD3CN, 300.13 MHz, δ ppm): 0.96 (t, 12H, CH3), 1.34 (m, 8H, CH3-CH2), 1.59 (m , 8H, CH2-CH2N), 3.06 (m, 8H, NCH2).19F NMR (25 °C, CD3CN, 300.13 MHz, δ ppm): -73.0 (d, 6F, PF6, JP-F = 706.7 Hz).31P NMR (25 °C, CD3CN, 300.13 MHz, δ ppm): - 144.6 (sept, 1P, PF6, JP-F = 706.7 Hz). Infrared spectrum (ATR, 32 scans, ν cm-1): 2966 (m), 2937 (w), 2879 (w), 1472 (m), 1404 (w), 1386 (w), 1360 (w), 1350 (w), 1319 (w), 1260 (w), 1242 (w), 1165 (w), 1109 (w), 1070 (w), 1035 (w), 931 (w), 880 (m), 829 (s), 738 (m), 555 (s).

1643-19-2
1065 suppliers
$12.00/10g

3109-63-5
302 suppliers
$5.00/5g
Yield:3109-63-5 88%
Reaction Conditions:
with potassium hexafluorophosphate in dichloromethane;water at 20; for 24 h;
Steps:
1 Preparation Description (1): Synthesis of [(n-Bu4)N] [PF6]
K[PF6] (2.055 g, 11.2 mmol) was dissolved in 30 ml of H20. [(n-Bu4)N]Br (3.601 g, 11.2 mmol) was dissolved in 30 ml of CH2C12 and added to the aqueous solution of K[PF6]. After stirring for 24 hours at ambient temperature the phases were separated. The organic phase was washed three times with 10 ml of water dried over anhydrous Na2C03 and filtered. The filtrate was concentrated on a rotary evaporator to obtain a white solid. The obtained solid was dried at 80°C in vacuum for 18 hours. The yield of [(n-Bu4)N][PF6] was 3.822 g (88 %, 9.87 mmol).DSC (10 K/min): m.p. = 250°C, Tdec = 388°C C/H/N Analysis calc. % (found): C 49.60 (49.97), H 9.37 (9.00), N 3.61 (3.61) 1H NMR (25°C, CD3CN, 300.13 MHz, delta in ppm): 0.96 (t, 12H, CH3), 1.34 (m, 8H, CH3-CH2), 1.59 (m, 8H, CH2-CH2N), 3.06 (m, 8H, NCH2) 19F NMR (25°C, CD3CN, 300.13 MHz, delta in ppm): -73.0 (d, 6F, PF6, ^P-^F) = 706.7 Hz) 31P NMR (25°C, CD3CN, 300.13 MHz, delta in ppm): -144.6 (sept, IP, PF6, ^P-^F) = 706.7 Hz) IR (ATR, 32 scans, v in cm"1): 2966 (m), 2937 (w), 2879 (w), 1472 (m), 1404 (w), 1386 (w), 1360 (w), 1350 (w), 1319 (w), 1260 (w), 1242 (w), 1165 (w), 1109 (w), 1070 (w), 1035 (w), 931 (w), 880 (m), 829 (s), 738 (m), 555 (s)
References:
LONZA LTD;SIEVERT, Katharina;SCHULZ, Axel;HARLOFF, Jörg;STOFFERS, Alrik;ELLINGER, Stefan;OTT, Lothar;RIJKSEN, Christiaan;FRANKE, Patrick;TAESCHLER, Christoph;ZUR TAESCHLER, Cornelia WO2014/167034, 2014, A2 Location in patent:Page/Page column 48; 49
1112-67-0
547 suppliers
$6.00/25g
762-04-9
356 suppliers
$10.00/10 g

3109-63-5
302 suppliers
$5.00/5g
2052-49-5
399 suppliers
$10.00/5g

3109-63-5
302 suppliers
$5.00/5g
17084-13-8
399 suppliers
$15.00/25g

1112-67-0
547 suppliers
$6.00/25g

3109-63-5
302 suppliers
$5.00/5g