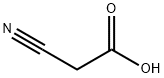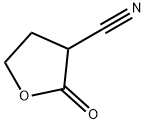
TETRAHYDRO-2-OXO-3-FURANCARBONITRILE synthesis
- Product Name:TETRAHYDRO-2-OXO-3-FURANCARBONITRILE
- CAS Number:27512-26-1
- Molecular formula:C5H5NO2
- Molecular Weight:111.1
Yield:27512-26-1 73%
Reaction Conditions:
Stage #1: ethyl 2-cyanoacetatewith potassium tert-butylate in tert-butyl alcohol at 20; for 1 h;
Stage #2: oxirane in ethanol at 45 - 50; for 3 h;Reagent/catalyst;Solvent;Temperature;
Steps:
2.1 (1) cyclization reaction
11.78 g (0.105 mol) of potassium tert-butoxide was dissolved in 250 ml four-necked flask and 50 ml of t-butanol was added. 11.3 g (0.1 mol) of ethyl cyanoacetate was added at 20 ° C and stirred at 20 ° C 1 hour, a large amount of white solid appears. 5.54 g (0.126 mol) of ethylene oxide was introduced into the four-necked flask at room temperature. After the introduction was complete, the temperature was raised to 45 to 50 ° C and stirred for 3 hours. The white solid disappeared and the system became amber clear solution.The whole system was subjected to vacuum distillation to remove t-butanol. For the distillation residue, 50 ml of purified water was added to the distillation residue and the pH was adjusted to 2 to 3 with concentrated hydrochloric acid and extracted with ethyl acetate (50 ml * , The organic phase was combined and the ethyl acetate was distilled off under reduced pressure. The residue was continued under reduced pressure. The organic phase was combined to give 8.1 g of a colorless clear oil of 3-cyano-γ-butyrolactone, 98% Yield 73%.
References:
CN106749117,2017,A Location in patent:Paragraph 0022; 0023; 0024; 0025; 0026; 0036-0039; 0046-0049

27512-24-9
8 suppliers
inquiry

27512-26-1
8 suppliers
inquiry