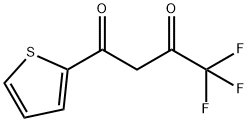
Thenoyltrifluoroacetone synthesis
- Product Name:Thenoyltrifluoroacetone
- CAS Number:326-91-0
- Molecular formula:C8H5F3O2S
- Molecular Weight:222.18
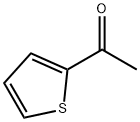
88-15-3
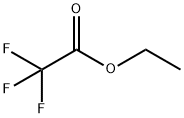
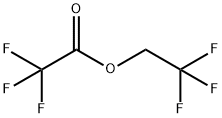
383-63-1

326-91-0
The general procedure for the synthesis of 2-thiophenecarbonyltrifluoroacetone from 2-acetylthiophene and ethyl trifluoroacetate was as follows: 1-(thiophen-2-yl)ethanone (20 g, 16 mmol) was added dropwise to a methanolic solution of sodium methanolate (10.3 g, 19 mmol), and the reaction was stirred for 1 hour at room temperature. Subsequently, the reaction mixture was cooled to 0 °C, ethyl 2,2,2-trifluoroacetate (27 g, 19 mmol) was added in batches and the whole reaction mixture was refluxed at 80 °C overnight. Upon completion of the reaction, the organic solvent was removed by vacuum evaporation and the residue was dissolved in water (200 mL), acidified with 1N hydrochloric acid (120 mL) and subsequently extracted three times with ethyl acetate (200 mL). The organic layers were combined, washed with brine, dried over anhydrous sodium sulfate, concentrated and further purified by silica gel column chromatography (petroleum ether/ethyl acetate, 20:1) to afford 4,4,4-trifluoro-1-(thiophen-2-yl)butane-1,3-dione (11 g, 32% yield) as a light red solid.
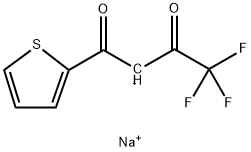
615-07-6
0 suppliers
inquiry
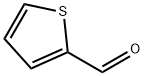
98-03-3
544 suppliers
$5.00/25g
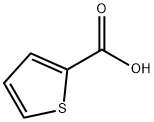
527-72-0
503 suppliers
$5.00/10g

326-91-0
284 suppliers
$5.00/1g
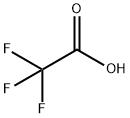
76-05-1
725 suppliers
$9.00/1g
Yield:-
Reaction Conditions:
with oxygen in acetonitrile; for 12 h;UV-irradiation;
Steps:
2.4. GC/MS analysis
50 mmol NaTTFA in acetonitrile (5 ml) mixed with K7050 (0.4 g/L)were illuminated by UV light (Philips CLEO 75 W) with an intensity of1mW cm- for 12 h in the presence of O2. Afterwards the mixture wasfiltrated in order to remove TiO2. The liquid extract was analyzed byGC/MS using a GCMS-QP5000 (Shimadzu) coupled with an AOC-5000Plus auto sampler and employing parameters based on Method 8270D(SW-846): Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS). A capillary RXI-5MS column(30m×0.32 mm, dF=1 μm, Restek) was used to perform the separationprocedure. In a typical analysis 2 μl of the liquid extract wereinjected in a split/splitless injector of the GC/MS system. The columnoven was held at 40 °C for 4 min, followed by an increase in a rate of10 °C/min to reach a final temperature of 270 °C. The temperature ofboth, the injection and the MS interface was adjusted to 280 °C. The MSscanned from 35 m/z to 500 m/z.
References:
Melchers, Stephanie;Alsalka, Yamen;Schneider, Jenny;Bahnemann, Detlef W. [Journal of Photochemistry and Photobiology A: Chemistry,2018,vol. 364,p. 303 - 308]