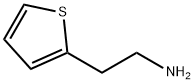
Thiophene-2-ethylamine synthesis
- Product Name:Thiophene-2-ethylamine
- CAS Number:30433-91-1
- Molecular formula:C6H9NS
- Molecular Weight:127.21
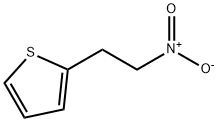
30807-46-6
0 suppliers
inquiry

30433-91-1
356 suppliers
$5.00/5g
Yield:30433-91-1 1009 g
Reaction Conditions:
with hydrogen at 120; for 12 h;Autoclave;
Steps:
1 Path B
The 10M2-chlorothiophene and 10M metal magnesium reagent format preparation, cooled to room temperature,Add 1000ml of anhydrous tetrahydrofuran, mix well,Slowly add 10M (the material optional range of 8-10M) nitroethylene,The reaction was refluxed for 3 hours (depending on the different substituted thiophene anion derivative,The reaction time is 3-12 hours, such as:The reaction time for the substitution of electron groups on the ring is 3-8 hours,The ring has a substitution of electron-withdrawing groups when the reaction time is 8-12 hours), the solvent was removed under reduced pressure stand-by.The above product was dissolved in 3000 ml of DMSO, 10M acetic acid was added dropwise and reacted at 120 ° C for 5 hours,Recrystallization gave 1231 g of product.Or the product was added 1MPd / C or Raney Ni, 10M of hydrogen was introduced in an autoclave, at120 ° C for 12 hours, filtered, extracted, and distilled under reduced pressure to obtain the product 1009g.
References:
Wang Zhixun CN106554343, 2017, A Location in patent:Paragraph 0039; 0040; 0041
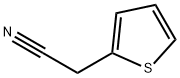
20893-30-5
197 suppliers
$9.00/1g

30433-91-1
356 suppliers
$5.00/5g
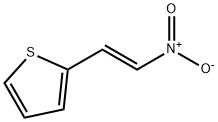
34312-77-1
128 suppliers
$20.00/5g

30433-91-1
356 suppliers
$5.00/5g
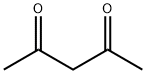
123-54-6
588 suppliers
$10.00/25ml

30433-91-1
356 suppliers
$5.00/5g
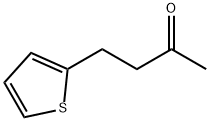
59594-93-3
2 suppliers
inquiry

30433-91-1
356 suppliers
$5.00/5g