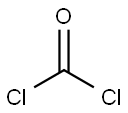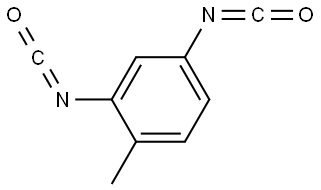
Tolylene-2,4-diisocyanate synthesis
- Product Name:Tolylene-2,4-diisocyanate
- CAS Number:584-84-9
- Molecular formula:C9H6N2O2
- Molecular Weight:174.16
Yield:584-84-9 99.5%
Reaction Conditions:
under 1050.11 Torr;Product distribution / selectivity;Gas phase;Industry scale;
Steps:
3
Example 3; According to the Invention2,500 kg/h of 2,4-toluenediamine (TDA for short) were vaporized and fed in gaseous form at a temperature of 410° C. to a cylindrical-conical tube reactor via a nozzle (diameter 110 mm) arranged on the reactor axis. At the same time, in parallel with this 8,097 kg/h of gaseous phosgene were heated to 390° C. and likewise fed to the tube reactor at the annular space left free by the one nozzle. The reactor had a diameter of 600 mm in the region of the educt feed. This diameter was retained to an axial position 350 mm downstream of the nozzle mouth, and thereafter a section with a conical expansion (expanding half-angle, i.e., angle between the reactor wall and reactor axis equal to 3.5°) followed. The streams were mixed in the tube reactor under an operating pressure of 1,400 mbar abs.. The reaction of the amine groups to give isocyanate groups started at the same time. 350 mm downstream of the nozzle mouth a conversion of the amine groups into isocyanate groups of 4% was reached. The gas volume stream started to increase significantly due to the stoichiometry and the reaction enthalpy, but as a result of the increasing flow cross-section, at no location lying downstream did the flow rate averaged over the cross-section exceed the starting rate averaged over the cross-section at the location of the stated conversion of the amine groups into isocyanate groups of 4%, i.e at a length of the tube, reactor of 350 mm. The course of the flow rate averaged over the cross-section with respect to the length of the tube reactor is shown in FIG. 1. It was found that the flow rate averaged over the cross-section was always lower from a conversion of the amine groups into isocyanate groups of 4%, i.e after a length of 350 mm from the nozzle mouth, than at the conversion of the amine groups into isocyanate groups of 4%, i.e at the length of 350 mm. At a 2,4-toluene-diisocyanate yield of >99.5%, a minimum reaction space length from the nozzle mouth of only 4.2 m was required before the reaction of the 2,4-toluenediamine and its secondary products with the phosgene had been to the greatest extent completely concluded.
References:
US2010/152484,2010,A1 Location in patent:Page/Page column 8
95-80-7
265 suppliers
$12.00/1mg

584-84-9
228 suppliers
$23.00/25g
4223-24-9
3 suppliers
$172.00/250mg

584-84-9
228 suppliers
$23.00/25g
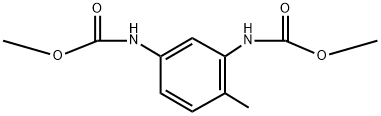
6935-99-5
76 suppliers
inquiry

584-84-9
228 suppliers
$23.00/25g
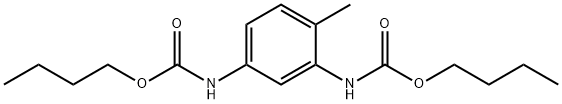
7469-49-0
3 suppliers
$172.00/250mg

584-84-9
228 suppliers
$23.00/25g