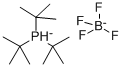
Tri-tert-butylphosphine tetrafluoroborate synthesis
- Product Name:Tri-tert-butylphosphine tetrafluoroborate
- CAS Number:131274-22-1
- Molecular formula:C12H28P.BF4
- Molecular Weight:290.13
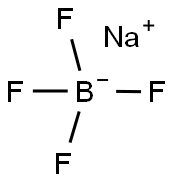
13755-29-8
495 suppliers
$6.00/25g
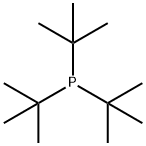
13716-12-6
326 suppliers
$45.00/500mg
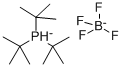
131274-22-1
328 suppliers
$6.00/1g
Yield:131274-22-1 87%
Reaction Conditions:
Stage #1: tri-tert-butyl phosphinewith hydrogenchloride in water at 20; for 1 h;
Stage #2: sodium tetrafluoroborate in water; for 1 h;Reagent/catalyst;
Steps:
1-5
After the reaction was completed, 30 mL of 36% aqueous hydrochloric acid solution and 50 mL of water were added to the reaction flask. The reaction solution was slowly poured into the above-mentioned aqueous hydrochloric acid solution and stirred at room temperature for 1 hour. Next, solid sodium tetrafluoroborate (13.1 g) was added, and the reaction was continued to stir for 1 hour. Ethyl acetate was added to separate the layers, the organic layer was washed with water and saturated brine, the organic layers were combined and dried over anhydrous sodium sulfate. After filtering, the organic layer was distilled under reduced pressure to obtain a sticky solid oily substance, which was added with 80 mL of ethanol and heated to reflux, filtered off a small amount of insoluble matter, and cooled to room temperature for crystallization. The obtained solid was filtered, washed with 20 mL of MTBE, and dried to obtain 25.3 g of white flaky solid, yield 87%
References:
CN113683639,2021,A Location in patent:Paragraph 0017; 0019-0020; 0022-0023; 0025-0026; ...

13716-12-6
326 suppliers
$45.00/500mg
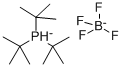
131274-22-1
328 suppliers
$6.00/1g
677-22-5
278 suppliers
$45.00/10ml
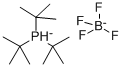
131274-22-1
328 suppliers
$6.00/1g