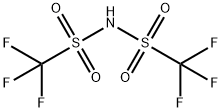
Trifluoromethanesulfonimide synthesis
- Product Name:Trifluoromethanesulfonimide
- CAS Number:82113-65-3
- Molecular formula:C2HF6NO4S2
- Molecular Weight:281.15
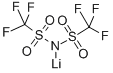
90076-65-6

82113-65-3
The general procedure for the synthesis of lithium bis(trifluoromethanesulfonyl)imide (HTf2N) from lithium bis(trifluoromethanesulfonyl)imide (LiTf2N) is as follows: LiTf2N is dissolved in concentrated sulfuric acid, followed by sublimation at 70 °C under reduced pressure (10^-3 mbar) to give the HTf2N crude product. This crude product can be further purified. The yield was 90%. The NMR hydrogen spectrum (1H-NMR, D2O) of the product showed: δ 4.77 (s, 1H); the NMR fluorine spectrum (19F-NMR, D2O) showed: δ -79.16 (s, 6F); and the NMR carbon spectrum (13C{19F}NMR, D2O) showed: δ 119.27 (s, 2C).
![Methanesulfonamide, 1,1,1-trifluoro-N-(phenylmethyl)-N-[(trifluoromethyl)sulfonyl]-](/CAS/20200515/GIF/35034-08-3.gif)
35034-08-3
0 suppliers
inquiry

82113-65-3
269 suppliers
$25.00/1g
Yield:82113-65-3 82%
Reaction Conditions:
with sulfuric acid in toluene at 0 - 20; for 1 h;
Steps:
7.2
2) Weigh 29.44g benzyl bistrifluoromethylsulfonamide was dissolved in 80mL of toluene, after cooling to 0 ° C,Slowly add 98% concentrated sulfuric acid 6.5g, the reaction time at room temperature for 1h, until the reaction is completed,100 ml of ice water and 100 ml of dichloromethane were added and neutralized with 2 mol / L of sodium hydroxide solution,The organic layer was collected, magnesium sulfate was added to the organic layer and dried, filtered,After the filtrate was evaporated under reduced pressure to remove methylene chloride,Recrystallization from 50 mL of n-hexane afforded 13.38 g of bistrifluoromethanesulfonamide,The yield (based on benzyl bistrifluoromethanesulfonamide) was 82%.
References:
CN105949093,2016,A Location in patent:Paragraph 0044; 0046; 0049; 0052; 0054

90076-65-6
460 suppliers
$6.00/5g

82113-65-3
269 suppliers
$25.00/1g
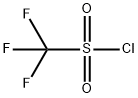
421-83-0
250 suppliers
$22.39/1G

82113-65-3
269 suppliers
$25.00/1g

421-83-0
250 suppliers
$22.39/1G
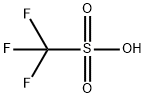
1493-13-6
659 suppliers
$12.00/5g

82113-65-3
269 suppliers
$25.00/1g

421-83-0
250 suppliers
$22.39/1G
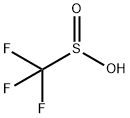
34642-42-7
1 suppliers
inquiry

1493-13-6
659 suppliers
$12.00/5g

82113-65-3
269 suppliers
$25.00/1g