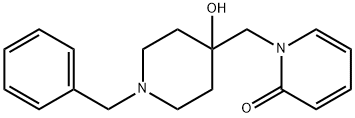
YL-0919 synthesis
- Product Name:YL-0919
- CAS Number:1339339-18-2
- Molecular formula:C18H22N2O2
- Molecular Weight:298.38
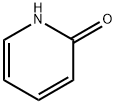
142-08-5
511 suppliers
$6.00/5g
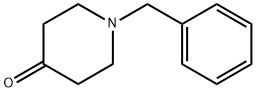
3612-20-2
443 suppliers
$6.00/10g
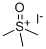
1774-47-6
551 suppliers
$6.00/25g

1339339-18-2
10 suppliers
inquiry
Yield:1339339-18-2 88.9 %
Reaction Conditions:
Stage #1: 1-benzyl-4-piperidone;trimethylsulfoxonium iodidewith tetra(n-butyl)ammonium hydrogensulfate;sodium hydroxide in toluene at 65;Large scale;
Stage #2: 2-hydroxypyridin in toluene;Large scale;Reagent/catalyst;Temperature;
Steps:
1-4 Example 4
At room temperature, weigh 70.0kg N-benzyl-4-piperidone, 89.5kg trimethylsulfoxide iodide, 6.3kg tetrabutylammonium bisulfate, 490L toluene and mix, then add 39.5kg 15% sodium hydroxide Alkaline solution, heat up to 65°C and keep it warm for 4 hours, then add 38.7kg 2-hydroxypyridine, continue to react for 12 hours, after the reaction is over, let it stand for stratification, add an appropriate amount of drinking water to wash the toluene phase, concentrate the toluene phase to dryness, add an appropriate amount of ethanol to raise the temperature Dissolve, slowly cool down to -10°C, filter, and dry to obtain white piperapidone with a yield of 88.9% and a purity of 99.3%.
References:
CN115433162,2022,A Location in patent:Paragraph 0037-0044

3612-20-2
443 suppliers
$6.00/10g

1339339-18-2
10 suppliers
inquiry

100-46-9
492 suppliers
$5.00/5G

1339339-18-2
10 suppliers
inquiry
![6-benzyl-1-oxa-6-azaspiro[2,5]octane](/CAS/GIF/19867-34-6.gif)