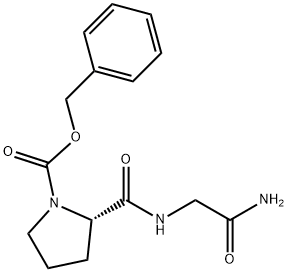
Z-PRO-GLY-NH2 synthesis
- Product Name:Z-PRO-GLY-NH2
- CAS Number:35010-96-9
- Molecular formula:C15H19N3O4
- Molecular Weight:305.33
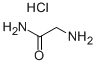
1668-10-6
509 suppliers
$5.00/5g
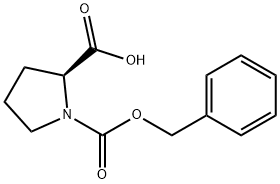
1148-11-4
455 suppliers
$6.00/10g

35010-96-9
38 suppliers
$32.00/250mg
Yield:35010-96-9 100%
Reaction Conditions:
with dmap;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in dichloromethane at 20;
Steps:
1
1-{[(Phenylmethyl)oxy]carbonyl}-L-proline (8.79 mmol, 2.19 g), glycinamide hydrochloride (8.79 mmol, 0.97 g), DMAP (26.38 mmol, 3.22 g), and EDCI (13.19 mmol, 2.54 g) were all dissolved in CH2Cb (80 ml_) at room temperature and stirred overnight. An additional 100 ml_ CH2CI2 was added, followed by 1 N HCI (100 ml_). The organic layer was separated, dried (Na2SO4), filtered, and evaporated to give the desired product as a white solid in quantitative yield.PIFA (4.85 mmol, 2.09 g) was dissolved in CH3CN (15 mL) and diluted with H2O (15 ml_). 1-{[(Phenylmethyl)oxy]carbonyl}-L-prolylglycinamide (4.8 mmol, 1.48 g) was added to this solution and the reaction mixture was stirred at room temperature overnight. It was then diluted with 1 N HCI (150 mL) and washed twice with diethyl ether. The aqueous layer was concentrated in vacuo to give the desired product in quantitative yield. The resulting white solid was used without further purification.Phenylmethyl (2S)-2-{[(aminomethyl)amino]carbonyl}-1-pyrrolidinecarboxylate hydrochloride (0.988 mmol, 0.312 g), 2-pyridinecarboxylic acid (0.988 mmol, 0.122 g), HOAT (1.186 mmol, 0.161 g), EDCI (1.186 mmol, 0.228 g), and NMM (9.88 mmol, 1.1 mL) were all dissolved in DMF (10 mL) and stirred at room temperature overnight. The crude reaction was purified by reverse phase HPLC to give a yellow oil (0.25 g, 66% yield).
References:
WO2007/67906,2007,A2 Location in patent:Page/Page column 24-25
61058-42-2
5 suppliers
$28.60/50MG

35010-96-9
38 suppliers
$32.00/250mg
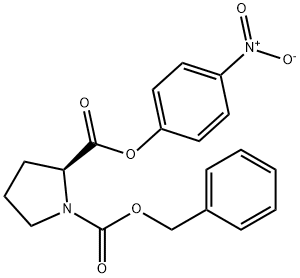
3304-59-4
80 suppliers
$11.65/5G

35010-96-9
38 suppliers
$32.00/250mg