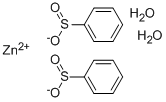
Zinc benzenesulfinate dihydrate synthesis
- Product Name:Zinc benzenesulfinate dihydrate
- CAS Number:24308-84-7
- Molecular formula:C12H14O6S2Zn
- Molecular Weight:383.76
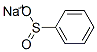
873-55-2
406 suppliers
$10.00/5g
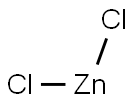
7646-85-7
605 suppliers
$10.00/25ml

24308-84-7
204 suppliers
inquiry
Yield:24308-84-7 99.4 mol
Reaction Conditions:
in water at 74; for 1.5 h;Temperature;
Steps:
1.S2; 2.S2; 3.S2; 4.S2 Example 4:
A method for synthesizing zinc benzenesulfinate includes the following steps: S1. Using benzenesulfonyl chloride as a starting material, sodium sulfite as a reducing agent, and water as a solvent, in the presence of an inorganic base, benzenesulfonyl chloride is reduced to a benzenesulfinate to obtain an aqueous solution of benzenesulfinate;S2. To the aqueous benzenesulfinate solution obtained in step S1, add an aqueous solution of zinc chloride to perform the reaction and stop the reaction; cool down, wash with water, and dry to obtain the target product zinc benzenesulfinate; In this embodiment, the reaction temperature in step S2 is 74 ° C. and the reaction time is 90 min.In this embodiment, in step S1, the molar ratio of benzenesulfonyl chloride to sodium sulfite is 1: 1.14. In this embodiment, in step S1, the inorganic base is sodium carbonate. In this embodiment, the reaction temperature in step S1 is 50 ° C .; when the pH of the reaction solution is 7.2, the reaction is stopped.In this embodiment, in step S2, the molar ratio of the benzenesulfinate to the zinc chloride aqueous solution is 1: 0.53.In this embodiment, in step S2, the temperature reduction is to reduce the temperature of the reaction solution to 32 ° C. after the reaction is completed.In this embodiment, in step S2, the drying temperature used for the drying is 70 ° C. In this embodiment, in step S2, the water is filtered and washed 4 times.The chemical reaction equation of this embodiment is as follows: In step S1 of this embodiment, the molar yield of benzenesulfinate is 93.3%; in step S2, the molar yield of zinc benzenesulfinate is 99.4%, and the purity by HPLC is 99.7%;
References:
CN110642759,2020,A Location in patent:Paragraph 0038-0090
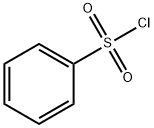
98-09-9
364 suppliers
$12.00/25g
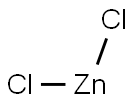
7646-85-7
605 suppliers
$10.00/25ml

24308-84-7
204 suppliers
inquiry