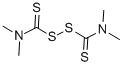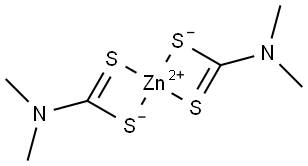
Zinc dimethyldithiocarbamate synthesis
- Product Name:Zinc dimethyldithiocarbamate
- CAS Number:137-30-4
- Molecular formula:C6H12N2S4Zn
- Molecular Weight:305.83
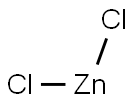
7646-85-7
605 suppliers
$10.00/25ml
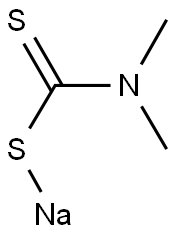
128-04-1
382 suppliers
$10.00/1g

137-30-4
241 suppliers
$10.00/1g
Yield:-
Reaction Conditions:
in water
Steps:
1 2.3.1.1. [Bis(dimethylcarbamodithioato-kS,kS′)zinc(II)]
General procedure: Solid ZnCl2 was added to an aqueous solution of sodium N,N-dimethyldithiocarbamate hydrate or ammonium pyrrolidinedithiocarbamate in molar ratio metal-to-ligand 1:2, under vigorous stirring leading to the immediate precipitation of a white solid.
The precipitate was filtered off, washed with water and n-pentane and dried in vacuum over P4O10, giving a final yield of 75-80%.Found: C, 23.72; H, 4.04; N, 9.12; S, 41.88. Calc. for C6H12ZnN2S4 (M.W. = 305.78 g/mol): C, 23.57; H, 3.96; N, 9.16; S, 41.94%) FT-IR (KBr or nujol, νmax/cm- 1): ν(N-CSS) = 1525 br; νa,s(SCS) = 974, 565; ν(ZnS) = 381. 1H NMR: δH (DMSO-d6, ppm): 3.38 (12H, s, CH3N).
13C NMR: δC (DMSO-d6, ppm): 44.64 (CH3-N); 203.72 (CS). EDS calculated element ratio Zn:S = 0.25.
References:
Nagy, Eszter Márta;Sitran, Sergio;Montopoli, Monica;Favaro, Monica;Marchiò, Luciano;Caparrotta, Laura;Fregona, Dolores [Journal of Inorganic Biochemistry,2012,vol. 117,p. 131 - 139]
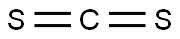
75-15-0
273 suppliers
$40.00/100 g

124-40-3
545 suppliers
$18.00/100ml
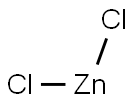
7646-85-7
605 suppliers
$10.00/25ml

137-30-4
241 suppliers
$10.00/1g