| Identification | More | [Name]
Nalidixic acid | [CAS]
389-08-2 | [Synonyms]
1,4-DIHYDRO-1-ETHYL-7-METHYL-1,8-NAPHTHYRIDIN-4-ONE-3-CARBOXYLIC ACID
1,4-DIHYDRO-1-ETHYL-7-METHYL-4-OXO-1,8-NAPHTHYRIDINE-3-CARBOXYLIC ACID
1-ETHYL-1,4-DIHYDRO-7-METHYL-4-OXO-1,8-NAPHTHYRIDINE-3-CARBOXYLIC ACID
1-ETHYL-7-METHYL-1,8-NAPHTHYRIDINE-4-ONE-3-CARBOXYLIC ACID
NALADIXIC ACID
NALIDIXIC ACID
NOGRAM
1,8-Naphthyridine-3-carboxylicacid,1-ethyl-1,4-dihydro-7-methyl-4-oxo-
1-aethyl-7-methyl-1,8-naphthyridin-4-on-3-karbonsaeure
1-Ethyl-1,4-dihydro-7-methyl-4-oxo-1,8-naphthyridine-3-carboxilicacid
1-ethyl-1,4-dihydro-7-methyl-4-oxo-8-naphthyridine-3-carboxylicacid
1-Ethyl-7-methyl-1,4-dihydro-1,8-naphthyridin-4-one-3-carboxylicacid
1-ethyl-7-methyl-1,8-naphthyridin-4-one-3-carboxylicacid
1-Ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylicacid
3-Carboxy-1-ethyl-7-methyl-1,8-naphthidin-4-one
3-carboxy-1-ethyl-7-methyl-1,8-naphthyridin-4-one
8-Naphthyridine-3-carboxylicacid,1-ethyl-1,4-dihydro-7-methyl-4-oxo-1
acide1-etil-7-metil-1,8-naftiridin-4-one-3-carbossilico
acidenalidixico
acidenalidixique | [EINECS(EC#)]
206-864-7 | [Molecular Formula]
C12H12N2O3 | [MDL Number]
MFCD00006884 | [Molecular Weight]
232.24 | [MOL File]
389-08-2.mol |
| Chemical Properties | Back Directory | [Appearance]
Crystalline Powder | [Melting point ]
227-229 °C (lit.) | [Boiling point ]
374.4°C (rough estimate) | [density ]
1.2243 (rough estimate) | [refractive index ]
1.6660 (estimate) | [storage temp. ]
0-6°C | [solubility ]
chloroform: 20 mg/mL, clear
| [form ]
Powder | [pka]
pKa 6.11± 0.02(Approximate) | [color ]
White to light yellow | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [Water Solubility ]
0.1 G/L (23 ºC) | [Usage]
Antibacterial | [Merck ]
14,6359 | [BRN ]
750515 | [CAS DataBase Reference]
389-08-2(CAS DataBase Reference) | [EPA Substance Registry System]
389-08-2(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R63:Possible risk of harm to the unborn child.
R42/43:May cause sensitization by inhalation and skin contact .
R40:Limited evidence of a carcinogenic effect.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S22:Do not breathe dust .
S36/37:Wear suitable protective clothing and gloves .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S24:Avoid contact with skin .
S36:Wear suitable protective clothing . | [WGK Germany ]
2
| [RTECS ]
QN2885000
| [F ]
8-10-23 | [HS Code ]
29339900 | [Safety Profile]
Poison by intravenous
and intraperitoneal routes. Moderately toxic
by ingestion and subcutaneous routes. An
experimental teratogen. Human systemic
effects: convulsions, hyperglycemia,
sweating, and blood changes in children.
Experimental reproductive effects.Questionable carcinogen with experimental
carcinogenic and tumorigenic data. Human
mutation data reported. Used as an
antibacterial agent and urinary tract
antiseptic. When heated to decomposition it
emits toxic fumes of NOx. | [Hazardous Substances Data]
389-08-2(Hazardous Substances Data) | [Toxicity]
LD50 in mice (mg/kg): 3300 orally; 500 s.c.; 176 i.v. (Lesher, 1962) |
| Hazard Information | Back Directory | [General Description]
Cream-colored powder. | [Air & Water Reactions]
Insoluble in water. | [Health Hazard]
SYMPTOMS: Ingestion of this material may cause nausea, vomiting, abdominal pain, allergic reactions and possible liver damage. | [Fire Hazard]
Flash point data for this chemical are not available, but NALIDIXIC ACID is probably combustible. | [Chemical Properties]
Crystalline Powder | [Originator]
Neggram,Winthrop,US,1964 | [Uses]
For the treatment of urinary tract infections caused by susceptible gram-negative microorganisms, including the majority of E. Coli, Enterobacter species, Klebsiella species, and Proteus species. | [Uses]
Nalidixic acid(NegGram) is a synthetic 1,8-naphthyridine antimicrobial agent with a limited bacteriocidal spectrum. It is an inhibitor of the A subunit of bacterial DNA gyrase. Evidence exists that the active metabolite, hydroxynalidixic acid, binds stron | [Uses]
Quinolone antibacterial. | [Definition]
ChEBI: A monocarboxylic acid comprising 1,8-naphthyridin-4-one substituted by carboxylic acid, ethyl and methyl groups at positions 3, 1, and 7, respectively. | [Manufacturing Process]
A warm solution containing 41 grams of 4-hydroxy-7-methyl-1,8-
naphthyridine-3-carboxylic acid and 39 grams of potassium hydroxide in 1
liter of ethanol and 200 cc of water was treated with 50 cc of ethyl iodide and
the resulting mixture was refluxed gently overnight, acidified with hydrochloric
acid and cooled. The resulting precipitate was collected and recrystallized
twice from acetonitrile to yield 26 grams (56% yield) of 1-ethyl-7-methyl-4-
oxo-1,8-naphthyridine-3-carboxylic acid, MP 229° to 230°C.
The starting material is prepared by reacting 2-amino-6-methylpyridine with
ethoxymethylene-malonic acid diethyl ester and then reacting that product
with sodium hydroxide. | [Brand name]
Neggram(Sanofi Aventis). | [Therapeutic Function]
Antibacterial | [Antimicrobial activity]
It displays good activity in vitro against a wide range of Enterobacteriaceae. | [Pharmaceutical Applications]
A 1,8 naphthyridone derivative available for oral administration. | [Pharmacokinetics]
Oral absorption: >90%
Cmax 1 g oral: c. 25 mg/L
Plasma half-life:c.1.5h
Volume of distribution :0.4 L/kg
Plasma protein binding: 93%
The plasma concentrations achieved after oral administration vary widely. In infants with acute shigellosis, absorption is much impaired by diarrhea. Administration with an alkaline compound leads to higher plasma concentrations, partly as the result of enhanced solubility (nalidixic acid is much more soluble at higher pH) and absorption and partly because of reduced tubular reabsorption.
It is rapidly metabolized, principally to the hydroxy acid, which is bacteriologically active, and glucuronide conjugates, which are not. The entire administered dose appears in the urine over a 24 h period. Elimination is reduced by probenecid. In the presence of renal impairment there is little accumulation of the active compound because it continues to be metabolized. However, elimination of metabolites is progressively delayed as renal function declines. About 4% of a dose appears in the feces. | [Clinical Use]
1-Ethyl-1,4-dihydro-7-methyl-4-oxo-1,8-naphthyridine-3-carboxylic acid (NegGram) occurs as a pale buff crystalline powder that is sparingly soluble in water and ether but solublein most polar organic solvents.Nalidixic acid is useful in the treatment of urinary tractinfections in which Gram-negative bacteria predominate.The activity against indole-positive Proteus spp. is particularlynoteworthy, and nalidixic acid and its congeners representimportant alternatives for the treatment of urinary tractinfections caused by strains of these bacteria resistant toother agents. Nalidixic acid is rapidly absorbed, extensivelymetabolized, and rapidly excreted after oral administration.The 7-hydroxymethyl metabolite is significantly more activethan the parent compound. Further metabolism of theactive metabolite to inactive glucuronide and 7-carboxylicacid metabolites also occurs. Nalidixic acid possesses at1/2elim of 6 to 7 hours. It is eliminated, in part, unchanged inthe urine and 80% as metabolites. | [Side effects]
Adverse reactions are generally those common to all quinolones: gastrointestinal tract and CNS disturbances and skin rashes, including eruptions related to photosensitivity. About half of the reported CNS reactions involve visual disturbances, hallucinations or disordered sensory perception. Severe excitatory states, including acute psychoses and convulsions, are usually observed in patients receiving high dosages. The drug should be avoided in patients with psychiatric disorders or epilepsy.
Acute intracranial hypertension has been observed in children, some of whom have also manifested cranial nerve palsies. Hemorrhage has occurred in patients who were also receiving warfarin, presumably due to displacement of the anticoagulant from its protein binding sites by the nalidixic acid. Hemolytic anemia has been described several times in infants with or without glucose-6-phosphate dehydrogenase deficiency; in adults, death has occurred from autoimmune hemolytic anemia. Arthralgia and severe metabolic acidosis have rarely been reported. | [Synthesis]
Nalidixic acid, 1-ethyl-1,4-dihydro-7-methyl-4-oxo-1,8-naphthiridin-3-
carboxylic acid (33.2.4), is synthesized by the following scheme. In the first stage, the
reaction of 2-amino-6-methylpyridine and diethyl ethoxymethylenemalonate forms the
substituted product (33.2.1), which when heated cyclizes to ethyl ester of 4-hydroxy
-7-methyl-1,8-napthiridin-3-carboxylic acid (33.2.2). Hydrolyzing the resulting product
with a base gives the corresponding acid (33.2.3). Alkylating this with ethyl iodide in the
presence of potassium hydroxide gives nalidixic acid.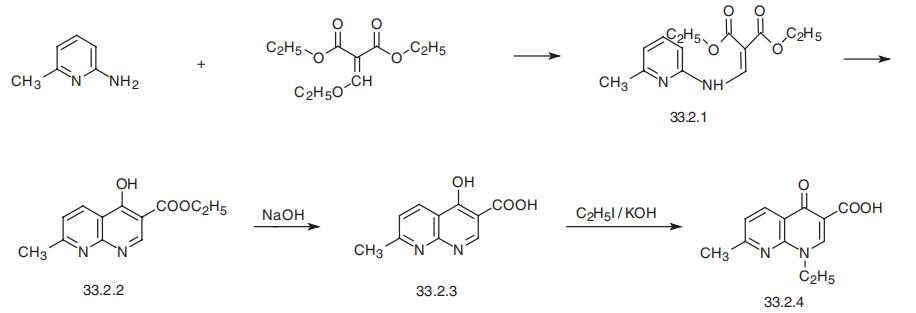
| [Drug interactions]
Potentially hazardous interactions with other drugs
Aminophylline and theophylline: possibly increased
risk of convulsions.
Analgesics: increased risk of convulsions with
NSAIDs.
Antibacterials: possibly antagonised by
nitrofurantoin.
Anticoagulants: anticoagulant effect of coumarins
enhanced.
Antimalarials: manufacturer of artemether with
lumefantrine advises avoid.
Ciclosporin: increased risk of nephrotoxicity.
Cytotoxics: increases risk of melphalan toxicity | [Metabolism]
Nalidixic acid is partially metabolised in the liver to
hydroxynalidixic acid, which has antibacterial activity
similar to that of nalidixic acid and accounts for about
30% of active drug in the blood. Both nalidixic acid and
hydroxynalidixic acid are rapidly metabolised to inactive
glucuronide and dicarboxylic acid derivatives; the major
inactive metabolite 7-carboxynalidixic acid is usually only
detected in urine. | [storage]
Store at -20°C | [Purification Methods]
Nalidixic acid crystallises from H2O or EtOH as a pale buff powder. It is soluble at 23o in CHCl3 (3.5%), toluene (0.16%), MeOH (0.13%), EtOH (0.09%), H2O (0.01%) and Et2O (0.01%). It inhibits nucleic acid and protein synthesis in yeast. [Lesher et al. J Med & Pharm Chem 5 1063 1962.] |
|
|