| Identification | More | [Name]
Glycerol | [CAS]
56-81-5 | [Synonyms]
GLYCERIN GEL LOADING DYE
LOADING BUFFER GLYCEROL
1,2,3-Propeatriol
1,2,3-Trihydroxyopropane
90 Technical glycerin
90 Technical glycerine
90technicalglycerine
Bulbold
citifluoraf2
Clyzerin, wasserfrei
clyzerin,wasserfrei
Cristal
Croderol G7000
Dagralax
Dynamite glycerin
Emery 912
Glyceol
Glycerin USP
glycerin(mist)
Glycerin, anhydrous | [EINECS(EC#)]
200-289-5 | [Molecular Formula]
C3H8O3 | [MDL Number]
MFCD00675440 | [Molecular Weight]
92.09 | [MOL File]
56-81-5.mol |
| Chemical Properties | Back Directory | [Appearance]
Glycerol is a viscous colorless or pale yellow,
odorless, syrupy liquid. | [Melting point ]
20 °C(lit.)
| [Boiling point ]
290 °C | [density ]
1.25 g/mL(lit.) | [vapor density ]
3.1 (vs air)
| [vapor pressure ]
<1 mm Hg ( 20 °C)
| [FEMA ]
2525 | [refractive index ]
n20/D 1.474(lit.)
| [Fp ]
320 °F
| [storage temp. ]
2-8°C
| [solubility ]
H2O: 5 M at 20 °C, clear, colorless
| [form ]
Viscous Liquid | [pka]
14.15(at 25℃) | [color ]
APHA: ≤10 | [Specific Gravity]
1.265 (15/15℃)1.262 | [Odor]
Odorless. | [PH]
5.5-8 (25℃, 5M in H2O) | [PH Range]
5.5 - 8 | [Stability:]
Stable. Incompatible with perchloric acid, lead oxide, acetic anhydride, nitrobenzene, chlorine, peroxides, strong acids, strong bases. Combustible. | [biological source]
synthetic (organic) | [explosive limit]
2.6-11.3%(V) | [Odor Type]
odorless | [Water Solubility ]
>500 g/L (20 ºC) | [Sensitive ]
Hygroscopic | [λmax]
λ: 260 nm Amax: 0.05
λ: 280 nm Amax: 0.04 | [JECFA Number]
909 | [Merck ]
14,4484 | [BRN ]
635685 | [Dielectric constant]
47.0(Ambient) | [Exposure limits]
OSHA: TWA 15 mg/m3; TWA 5 mg/m3 | [InChIKey]
PEDCQBHIVMGVHV-UHFFFAOYSA-N | [LogP]
-2.32 | [Uses]
glycerin (glycerol,56-81-5; propanetriol) is a humectant used in moisturizers. It is water-binding and able to draw and absorb water from the air, thus helping the skin retain moisture. glycerin has been studied extensively for its hydrating abilities. Based on the data available, glycerin has been established as a good skin-moisturizing agent. At least part of its activity is attributed to its facilitating enzymatic reactions in the skin, thereby promoting corneocyte desquamation. glycerin also improves the spreading qualities of creams and lotions. It is a clear, syrupy liquid made by chemically combining water and fat that is usually derived from vegetable oil. Although glycerin has not been shown to cause allergies, it may be comedogenic and irritating to the mucous membranes when used in concentrated solutions.
| [CAS DataBase Reference]
56-81-5(CAS DataBase Reference) | [NIST Chemistry Reference]
1,2,3-Propanetriol(56-81-5) | [EPA Substance Registry System]
56-81-5(EPA Substance) | [Absorption]
≤0.02 at 280nm in H2O at 0.5M
≤0.07 at 260nm in H2O at 0.5M |
| Safety Data | Back Directory | [Hazard Codes ]
F,Xn | [Risk Statements ]
R36:Irritating to the eyes.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R11:Highly Flammable. | [Safety Statements ]
S24/25:Avoid contact with skin and eyes .
S39:Wear eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [RIDADR ]
UN 1282 3/PG 2 | [WGK Germany ]
1
| [RTECS ]
MA8050000
| [F ]
3 | [Autoignition Temperature]
698 °F | [TSCA ]
Yes | [HS Code ]
29054500 | [Safety Profile]
Poison by
subcutaneous route. Mildly toxic by
ingestion. Human systemic effects by
ingestion: headache and nausea or vomiting.
Experimental reproductive effects. Human
mutation data reported. A skin and eye
irritant. In the form of mist it is a nuisance
particulate and inhalation irritant.
Combustible liquid when exposed to heat,
flame, or powerful oxidizers. Mixtures with hydrogen peroxide are highly explosive.
Ignites on contact with potassium
permanganate, calcium hypochlorite.
Mixture with nitric acid + sulfuric acid
forms the explosive glyceql nitrate. Mixture
with perchloric acid + lead oxide forms
explosive perchlorate esters. Confined
mixture with chlorine explodes if heated to
70-80'. Can react violently with acetic
anhydride, aniline + nitrobenzene, Ca(OCl)2,
Cr03,Cr203, F2 + PbO, phosphorus
triiodide, ethylene oxide + heat, KMnO4,
K2O2, AgClO4, Na2O2, NaH. Energetic
reaction with sodium hydride. Mixture with
nitric acid + hydrofluoric acid is a storage
hazard due to gas evolution. To fight fire,
use alcohol foam, CO2, dry chemical. When
heated to decomposition it emits acrid
smoke and fumes. | [Hazardous Substances Data]
56-81-5(Hazardous Substances Data) |
| Hazard Information | Back Directory | [General Description]
A colorless to brown colored liquid. Combustible but may require some effort to ignite. Residual sodium hydroxide (lye) causes crude material to be corrosive to metals and/or tissue. | [Reactivity Profile]
GLYCERINE is incompatible with strong oxidizers. GLYCERINE, [CRUDE, CONCENTRATED] is also incompatible with hydrogen peroxide, potassium permanganate, nitric acid + sulfuric acid, perchloric acid + lead oxide, acetic anhydride, aniline + nitrobenzene, Ca(OCl)2, CrO3, F2 + PbO, KMnO4, K2O2, AgClO4 and NaH. A mixture with chlorine explodes if heated to 158-176° F. GLYCERINE, [CRUDE, CONCENTRATED] reacts with acetic acid, potassium peroxide, sodium peroxide, hydrochloric acid, (HClO4 + PbO) and Na2O2. Contact with potassium chlorate may be explosive. GLYCERINE, [CRUDE, CONCENTRATED] also reacts with ethylene oxide, perchloric acid, nitric acid + hydrofluoric acid and phosphorus triiodide. | [Air & Water Reactions]
Hygroscopic. Water soluble. | [Health Hazard]
No hazard | [Potential Exposure]
Glycerol(56-81-5) is used as a humectant in
tobacco; it is used in cosmetics, antifreezes and inks. It is
used as a fiber lubricant. It is used as a raw material for
alkyd resins and in explosives manufacture.
| [Fire Hazard]
This chemical is combustible. | [First aid]
Move victim to fresh air. Call 911 or emergency
medical service. Give artificial respiration if victim is not
breathing. Do not use mouth-to-mouth method if victim
ingested or inhaled the substance; give artificial respira tion with the aid of a pocket mask equipped with a one-way
valve or other proper respiratory medical device.
Administer oxygen if breathing is difficult. Remove and
isolate contaminated clothing and shoes. In case of contact
with substance, immediately flush skin or eyes with running water for at least 20 minutes. For minor skin con tact, avoid spreading material on unaffected skin. Keep vic tim warm and quiet. Effects of exposure (inhalation,
ingestion, or skin contact) to substance may be delayed.
Ensure that medical personnel are aware of the material(s)
involved and take precautions to protect themselves.
Medical observation is recommended for 24 to 48 hours
after breathing overexposure, as pulmonary edema may be
delayed. As first aid for pulmonary edema, a doctor or
authorized paramedic may consider administering a drug or
other inhalation therapy. | [Shipping]
UN1760 Corrosive liquids, n.o.s., Hazard class:
8; Labels: 8-Corrosive material, Technical Name Required. | [Incompatibilities]
Able to polymerize above 300 ℉/150 ℃.Incompatible with acetic anhydrides (especially in the pres ence of a catalyst), strong acids, caustics, aliphatic amines,
and isocyanates. Strong oxidizers, e.g., chromium trioxide,
potassium chlorate, and potassium permanganate); can
cause fire and explosion hazard. Hygroscopic (i.e., absorbs moisture from the air). Decomposes when heated, produc ing corrosive gas of acrolein. | [Description]
Glycerol(56-81-5) is a colorless, viscous, hygroscopic, sweet-tasting trihydric alcohol. It is also called
glycerin or glycerine, with the term glycerol being preferred as the pure chemical form and
the term glycerin(e) being primarily used when the compound is used commercially in various
grades.
| [Chemical Properties]
Glycerin(56-81-5) is the polyhydric alcohol 1,2,3 propanetriol [HOCH2-CH(OH)CH2OH] also known as glycerol. A clear, colorless, syrupy
liquid having a sweet taste. It has not more than a slight
characteristic odor, which is neither harsh nor disagreeable. It is
hygroscopic and its solutions are neutral. Glycerin is miscible with
water and with alcohol. It is insoluble in chloroform, in ether, and
in fixed and volatile oils.
In the animal body, glycerin may be formed from ingested carbohydrates,
from glycogen by glycolysis, and from fats and other
lipids by hydrolysis. Commercially, glycerin can be produced by
a number of methods including microbial fermentation of sugars,
as a by-product in the manufacture of soap, or by synthesis from
propylene.
Animal and vegetable fats contain about 10 percent by weight of
glycerin. It is present in animal tissues to the extent of about
1 percent of the body weight. Glycerin is not an essential nutrient,
but it furnishes energy by contributing to the general pool of
oxidizable organic compounds.
| [Chemical Properties]
Glycerol(56-81-5) is a sweet-tasting, syrupy liquid It has not more than a slight characteristic odor, which is neither harsh nor disagreeable Glycerol is a trihydric alcohol It is hygroscopic and its solutions are neutral.
| [Chemical Properties]
Glycerol(56-81-5) is a viscous colorless or pale yellow,
odorless, syrupy liquid.
| [Chemical Properties]
Glycerol(56-81-5),CH20HCHOHCH20H, also known as glycerin and glycyl alcohol, is a clear, colorless, viscous liquid with a sweet taste.It is the simplest trihydroxy alcohol and a valuable chemical intermediary, It is soluble in water and alcohol, but only partially soluble in ether and ethyl acetate. Glycerol is used in perfume and medicine,as an antifreeze,and in manufacturing soaps and explosives.
| [Chemical Properties]
Glycerin is a clear, colorless, odorless, viscous, hygroscopic liquid; it
has a sweet taste, approximately 0.6 times as sweet as sucrose. | [Waste Disposal]
Mixture with a more flamma ble solvent followed by incineration. | [Occurrence]
Reported found in cocoa, apple, cider, beer, sour cherries, peach and wine | [History]
Glycerol(56-81-5) was first isolated from olive oil and lead oxide by the Swedish chemist
Carl Scheele (1742–1786) while making lead plaster soap in 1779. Scheele eventually realized
that glycerol was a common ingredient in fats and oils and referred to glycerol as “the
sweet principle of fats.” In 1811, the French chemist Michel Eugene Chevreul (1786–1889),
who was a pioneer in the study of fats and oils, proposed the name glycerine after the Greek
word glucos, which means sweet. Chevreul decomposed soaps isolating different acids such
as stearic and butyric acid and discovered that glycerol was liberated when oils and fats were
boiled in a basic mixture. Th éophile-Jules Pelouze (1807–1867) derived glycerol’s empirical
formula in 1836.
| [Definition]
ChEBI: A triol with a structure of propane substituted at positions 1, 2 and 3 by hydroxy groups. | [Production Methods]
Glycerol(56-81-5) is a by-product in the production of candles and soaps and was originally discardedin the production of these items. The process of converting a fat to soap is termedsaponification. The traditional method of saponification involved the use of animal fats andvegetable oils. Fats and oils are esters formed when three fatty-acid molecules attach to a singleglycerol molecule. When the three fatty acids attach to the three hydroxyl groups of the glycerol,a triglyceride is formed. During saponification of animal and plant products, hydrolysisof triglycerides converts triglycerides back to fatty acids and glycerol.the fatty acids then reactwith a base to produce a carboxylic acid salt commonly called soap.
Until 1940, the world’s demand for glycerol was supplied from natural sources throughthe production of soaps and candles. Glycerol can also be produced through the fermentationof sugar, and this process was used to increase glycerol production during World War I.Glycerol can also be produced synthetically from propylene. The synthetic production frompropylene first occurred just before World War II and commercial production started in 1943in Germany. The synthetic process begins with the chlorine substitution of one hydrogenatom of propylene to allyl chloride: H2C = CH-CH3 + Cl2 → H2C = CH-CH2Cl + HCl. Allylchloride is then treated with hypochlorous acid to produce 1,3-dichlorohydrin.
| [Production Methods]
Glycerin is mainly obtained from oils and fats as a by-product in the
manufacture of soaps and fatty acids. It may also be obtained from
natural sources by fermentation of, for example, sugar beet
molasses in the presence of large quantities of sodium sulfite.
Synthetically, glycerin may be prepared by the chlorination and
saponification of propylene. | [Reactions]
Glycerol reacts (1) with phosphorus pentachloride to form glyceryl trichloride, CH2Cl·CHCl · CH2Cl, (2) with acids to form esters, e.g., glycerol monoacetate CH2OH ·CHOH·CH2OOCCH3, glycerol diacetate C3H5(OH)(OCOCH3)2, glycerol triacetate (triacetin), CH2OOCCH3·CHOOCCH3·CH2OOCCH3, glycerol mononitrates (alpha, CH2OH·CHOH·CH2ONO2; beta, CH2OH · CHONO2·CH2OH), glycerol dinitrates (1, 2,CH2OH· CHONO2·CH2 ONO2; 1, 3,CH2ONO2·CHOH·CH2ONO2), glyceryl trinitrate (“nitroglycerine”), CH2ONO2·CHONO2·CH2ONO2, glyceryl tristearate (tristearin), CH2OOCC17H35·CHOO-CC17H35·CH2OOCC17H35, indirectly, glycerol monophosphates (alpha, CH2OH·CHOH·CH2OPO(OH)2, beta, CH2OH·CHOPO(OH)2·CH2OH, (3) with oxidizing agents, e.g., dilute nitric acid, to form glyceric acid, CH2OH·CHOH·COOH, tartaric acid, COOH·CHOH·COOH, mesoxalic acid, COOH·CO·COOH, (4) with phosphorus plus iodine, to form allyl iodide, CH2 : CHCH2I, which with hydrogen iodide yields propylene, CH2 : CHCH3, and then iso-propyl iodide, CH3CHICH3, (5) with sodium or sodium hydroxide to form alcoholates, (6) with sodium hydrogen sulfate or phosphorus pentoxide heated, to form acrolein, CH2 : CHCHO. Glycide alcohol is obtained by treatment of glycerol alphamonochlorohydrin CH2OH·CHOH·CH2Cl, which is made by reaction of hypochlorous acid and allyl alcohol with barium hydroxide. With hydrogen chloride, glycide alcohol yields epichlorohydrin. | [Aroma threshold values]
Greater than 20,000 ppm. | [Synthesis Reference(s)]
Tetrahedron Letters, 32, p. 187, 1991 DOI: 10.1016/0040-4039(91)80850-6 | [Chemical Reactivity]
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. | [Pharmaceutical Applications]
Glycerin(56-81-5) is used in a wide variety of pharmaceutical formulations
including oral, otic, ophthalmic, topical, and parenteral preparations.
In topical pharmaceutical formulations and cosmetics, glycerin is
used primarily for its humectant and emollient properties. Glycerin
is used as a solvent or cosolvent in creams and emulsions.
Glycerin is additionally used in aqueous and nonaqueous gels and
also as an additive in patch applications. In parenteral
formulations, glycerin is used mainly as a solvent and cosolvent.
In oral solutions, glycerin is used as a solvent, sweetening
agent, antimicrobial preservative, and viscosity-increasing agent. It
is also used as a plasticizer and in film coatings.
Glycerin is used as a plasticizer of gelatin in the production of
soft-gelatin capsules and gelatin suppositories.
Glycerin is employed as a therapeutic agent in a variety of
clinical applications, and is also used as a food additive.
| [Biochem/physiol Actions]
Glycerol(56-81-5) is hygroscopic in nature and is soluble in water owing to its three hydrophilic alcoholic hydroxyl groups. It can form both inter- and intramolecular hydrogen bonds, making it a very flexible molecule. The physiologic effect of glycerine is due to cell-mediated immunity, increased IgG production and increased histamine release.
| [Safety]
Glycerin(56-81-5) occurs naturally in animal and vegetable fats and oils that
are consumed as part of a normal diet. Glycerin is readily absorbed
from the intestine and is either metabolized to carbon dioxide and
glycogen or used in the synthesis of body fats.
Glycerin is used in a wide variety of pharmaceutical formulations
including oral, ophthalmic, parenteral, and topical preparations.
Adverse effects are mainly due to the dehydrating properties
of glycerin.
Oral doses are demulcent and mildly laxative in action. Large
doses may produce headache, thirst, nausea, and hyperglycemia.
The therapeutic parenteral administration of very large glycerin
doses, 70–80 g over 30–60 minutes in adults to reduce cranial
pressure, may induce hemolysis, hemoglobinuria, and renal failure.(
16) Slower administration has no deleterious effects.
Glycerin may also be used orally in doses of 1.0–1.5 g/kg bodyweight
to reduce intraocular pressure.
When used as an excipient or food additive, glycerin is not
usually associated with any adverse effects and is generally regarded
as a nontoxic and nonirritant material.
LD50 (guinea pig, oral): 7.75 g/kg
LD50 (mouse, IP): 8.70 g/kg
LD50 (mouse, IV): 4.25 g/kg
LD50 (mouse, oral): 4.1 g/kg
LD50 (mouse, SC): 0.09 g/kg
LD50 (rabbit, IV): 0.05 g/kg
LD50 (rabbit, oral): 27 g/kg
LD50 (rat, IP): 4.42 g/kg
LD50 (rat, oral): 5.57 g/kg
LD50 (rat, oral): 12.6 g/kg
LD50 (rat, SC): 0.1 g/kg
| [Synthesis]
Obtained from oils and fats as a by-product in the manufacture of soaps and fatty acids; synthesized from propylene; also production from sugars by fermentation. | [Environmental Fate]
Glycerol is completely miscible with water. When exposed to
moist air, it absorbs water (hydroscopic) as well as gasses such
as hydrogen sulfide and sulfur dioxide. Glycerol has low
volatility, with a vapor pressure of 0.000106 hPa at 25 ℃; the
calculated Henry’s law constant (maximum solubility) is
9.75E-6 Pam3 mol-1. The calculated photodegradation halflife
of glycerol in air is 6.8 h. Glycerol is readily biodegradable.
When released to the environment, glycerol is distributed
to water, with negligible amounts distributed in air, soil,
or sediment. Based on a log Kow of -1.76, glycerol has
a low bioaccumulation potential and is not expected to
bioaccumulate. | [Toxicity evaluation]
The medicinal actions of glycerol(56-81-5) are due to osmotic action;
orally, 75 ml of glycerol is equivalent to 996 mosmol. Large
intravenous doses of glycerol can cause hemolysis, hemoglobinuria,
and subsequent renal failure. The osmotic effect
of glycerol can lead to tissue dehydration and decreased cerebrospinal fluid pressure. Certain medical conditions
such as cardiac, renal, or liver disease and/or diabetes
mellitus may be exacerbated by shifts in body water as
a result of oral, intravenous, or rectal (suppositories)
administration of glycerol. No systemic effects have been
reported following the application of large amounts of
glycerol to the skin.
| [Toxics Screening Level]
The ITSL for glycerin is 100 μg/m3 based on an 8 hour averaging time. | [Regulatory Status]
GRAS listed. Accepted for use as a food additive in Europe.
Included in the FDA Inactive Ingredients Database (dental pastes;
buccal preparations; inhalations; injections; nasal and ophthalmic preparations; oral capsules, solutions, suspensions and tablets; otic,
rectal, topical, transdermal, and vaginal preparations). Included in
nonparenteral and parenteral medicines licensed in the UK.
Included in the Canadian List of Acceptable Non-medicinal
Ingredients. |
| Questions And Answer | Back Directory | [Physical and chemical properties]
Pure glycerol appears as colorless, odorless and sweet viscous liquid. Boiling point: 290 ° C, melting point: 17.9 ° C, the relative density: 1.2613. It can be miscible with water infinitely. It can be dissolved in 11 times ethyl acetate, about 500 times ether. It is insoluble in benzene, chloroform, carbon tetrachloride, carbon disulfide, petroleum ether and oil. Anhydrous glycerol has a strong water absorption property.
Glycerol is weakly acidic, being able to react with alkaline hydroxide. For example, it reaction with copper hydroxide can produce bright blue cupric glycerinate (can be used to identify polyols). Glycerol can react with nitric acid to generate glyceryl trinitrate, also known as nitroglycerin, being a strong explosive.
Because glycerol has water absorption property, it is often used as the moisturizing agent of cosmetics, leather, tobacco, food and textile. Glycerol also has effect on lubricating the intestine, being able to be used for enema or suppository treatment of constipation. Nitroglycerides have the effect of dilating coronary arteries and can be used to treat angina. Nitroglycerin can be used as an explosive and propellant. Glycerol can react with binary acid to generate alkyd resin, widely being used in paints and coatings.
In nature, glycerol is widely presented in the form of esters. For example, a variety of animal and vegetable oils are glycerol carboxylate with hydrolyzing grease being capable to generate fatty acids and glycerol. At present, one of the major sources of glycerol is the byproduct of the soap industry (grease is hydrolyzed under alkaline conditions). The other major source is from petroleum pyrolysis gas, propylene.
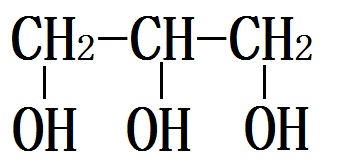
Figure 1 the glycerol structure. | [Pharmacological effects]
This product can lubricate and stimulate the intestinal wall, soften the stool, making it easy to discharge. It also has dehydration effect. When formulated together with the sodium ascorbate as compound injection for intravenous administration, it can reduce the intraocular pressure. Its topical administration has moisture absorption property, and can soften the local tissue. It can dissolve borax, boric acid, phenol, nucleic acid, salicylic acid and so on. It is mainly used for the treatment of constipation of children, the elderly and weak, the rescue of general brain edema, treatment of glaucoma, chapped and stripped winter skin and so on. | [Usage and Dosage]
Oral: 0.5~1g/kg per time; 1 or 2 times per day; dubbed into the 50% saline solution. It can be used in combination during the intermittent periods of other dehydration drug. Intravenous injection or intravenous infusion: 1g/kg per time once a day, it can be dubbed into 10% glycerol or glycerol saline solution. | [Adverse reactions]
This product is non-toxic with large dose of oral administration being able to cause headaches, dizziness, thirst, nausea, vomiting and diarrhea, but the symptoms are mostly mild and can disappears after bed rest. | [Precautions]
Diabetes patients should take with caution. High-concentration intravenous infusion (more than 30%) can cause hemolysis and hemoglobinuria. This product has hemolytic effect. During intravenous administration, avoid singly using this product, should instead combine with glucose or sodium chloride injection. | [Preparation and specifications]
Oral preparations: 50% glycerol; 0.9% sodium chloride solution; Injection: 9.263% glycerol 0.834% sodium chloride injection (glycerol sodium chloride injection). Medical glycerol suppository: obtained by absorbing glycerol using sodium stearate (soap) as a hardener. It contains about 90% glycerol with large type having a weight of 3 g and small type having a weight of about 1.5g. Glycerol solution: 10% glycerol sodium chloride solution, 10% glycerol glucose solution, 10% mannitol solution and 50% glycerol saline solution. | [Glycerol esterification]
Glycerol and fatty acids (saturated and unsaturated) are esterified to produce glycerides. The hydroxyl group can be subject to stepwise esterification during the reaction, forming (OH) 2 (OCOR), glycerol diester C3H5 (OH) (OCOR) 2 and triglyceride C3H5 (OCOR) 3.
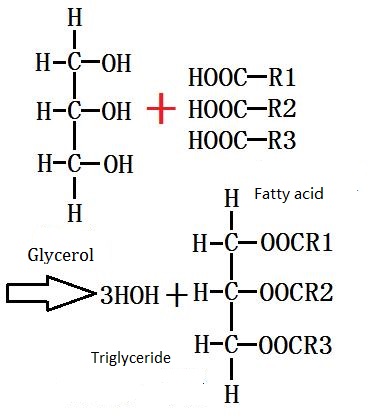
Figure 2 shows the esterification of glycerol.
It is an important method to prepare glycerol monoester and diglyceride in industry by direct reaction with oleyl ester and glycerol to obtain a mixture of mono-, diglyceride and triglyceride, and then separate the glycerol monoester by distillation. The method can prepare a glycerol monoester having a purity of 90%. During the experiment, the fatty acid, through the acid chloride, can react with glycerol to produce glycerides.
Glycerol and inorganic acids can also have esterification reaction. The most important reaction is with nitric acid. Under the conditions of stringent cooling, glycerol is added drop wise to the mixed acid of concentrated nitric acid and concentrated sulfuric acid to form glyceryl trinitrate (see "Nitroglyceride").
This information is edited by Xiaonan from ChemicalBook. | [Content analysis]
Preparation of sodium periodate solution: 60 g of sodium periodate (NaIO4) was dissolved in an aqueous solution containing 120 mL of 0.1 mol/L sulfuric acid. The volume is adjusted to 1000 ml with water. If the solution is not clear, then filter it through a sintered glass funnel. The solution is stored in a shade container with a glass stopper. The suitability of the test solution was tested as follows.
Draw 10ml into a 250ml volumetric flask, and mix with water to set the volume. Take about 550 mg of glycerol to dissolve in 50ml of water. Use a pipette to add 50 mL of the above-mentioned dilute periodic acid solution 50 mL. Take another 50 ml of dilute periodic acid solution and add to a flask containing 50 ml of water as a control. Each solution was allowed to stand for 30 mins and 5 ml of hydrochloric acid and 10 ml of potassium iodide test solution (TS-192) were added and mixed. And then let stand for 5min, add water 100ml, use 0.1mol/L sodium thiosulfate liquid for titration, constantly shake and add a few drops of starch solution (TS-235) upon being close to the end, continue to titrate to the end.
The ratio of the volume of 0.1 mol/L sodium thiosulfate consumed in the glycerol/periodate mixture over the blank test solution is applicable at the range of 0.750 and 0.765.
Operation: accurately weigh about 400 mg of sample and place into a 600ml beaker, add 50 ml of water for dilution, adding a few drops of bromine thymol test solution (TS-56) and acidify the 0.2mol/L sulfuric acid to obviously green or greenish yellow. Use 0.05 mol/L sodium hydroxide to neutralize to the clear blue end point (no green). On the other hand, take 50 mL of water for neutralization according to the above operation as the blank test. 50 ml of the sodium periodate solution was aspirated with a pipette, added to each beaker, slowly shaken and mixed, and covered with a surface dish and placed in a dark place or at room temperature (no more than 35 ° C) for 30 min. Add 10% of the mixture of equal volume of ethylene glycol and water, and then let stand for 30min. The solutions were separately diluted with water to about 300 ml.
With the help of a pH meter pre-calibrated with a pH of 4.0 phthalate (salt) standard buffer solution, titrate the sample solution with 0.1 mol/L sodium hydroxide to pH8.1士0.1 and titrate the blank sample to pH 6.5 ± 0.1. Each mL of 0.1mol/L sodium hydroxide is equivalent to 9.210mg of glycerol (C3H8O3) corrected by the blank sample. | [Toxicity]
ADI values are not subject to special provisions (FAO/WHO, 2001).
LD50:25g/kg (rat, oral).
It can be hydrolyzed, oxidized into nutrients inside the body. Even inhalation of 100 g dilute solution is also harmless. But a large amount of it can produce the ethanol-like anesthesia effect, and lead to high blood sugar.
GRAS (t} DA, § 182.1320, 2000); | [Usage limit]
FAO/WHO (1984, g/kg): Gotha cheese 5; edible ice and ice drinks 50.
FEMA (mg/kg): soft drink 570; cold drink 500; candy 980; baked goods 1300; pudding class 360; glue sugar 17~6000; meat 40; cake blooming 23000. | [Application]
It is a raw material for the production of nitroglycerin, acetic acid glycerol, surfactants, flavors, alkyds and ester gums. It can be directly used in antifreeze, cosmetics, inks, etc.
It can be used as water-retaining agent (used for bread and cake); carrier solvent (used in flavor, pigment and water-soluble preservative; thickener (used for drink and wine formulation); plasticizer (candy, desert and meat product); sweetener, gas chromatographic fixative.
EEC provides it can be used for alcoholic beverages, candy, cakes, coating glazing, meat and cheese coating, alcoholic beverages, bakery products, gelatin, gelatin and other sweets.
Glycerol(56-81-5) is used both in sample preparation and gel formation for polyacrylamide gel electrophoresis. Glycerol (5-10%) increases the density of a sample so that the sample will layer at the bottom of a gel sample well. Glycerol is also used to aid in casting gradient gels and as a protein stabilizer and storage buffer component. Glycerol is an important basic organic raw material, being widely used in industrial, pharmaceutical and daily life. There are about 1700 kinds of applications, mainly for medicine, cosmetics, alkyd resin, tobacco, food, sour resin, explosives, textile printing and dyeing and so on. The demand of glycerol in alkyd resin, celluloid and explosives exhibits a downward trend. But its demand in medicine, cosmetics and food will continue to grow. In previous years, the compositions of glycerol consumption in China include: 35.7% in paint; 32.6% in toothpaste; 4.8% in cosmetics; 6% in cigarettes, 5.9% in medicine, 4.8% in polyether and 10.2% for the other. During the manufacture of drugs and cosmetics, glycerol is widely used to prepare a variety of preparations, solvents, moisture, antifreeze and sweeteners. The cyclization of glycerol and p-nitroaniline can generate 6-nitroquinoline. The monostearate obtained from the acidification between glycerol and stearic acid is a kind of excipient, used as the matrix of hydrophilic ointment. Glycerol can generate acrolein by elimination reaction, and has been used to produce methionine and glutaraldehyde. The potassium glycerol phosphate, sodium glycerophosphate, calcium glycerophosphate made from glycerol and phosphoric acid are all used as a nutritional medicine. Chlorination of glycerol can generate the intermediate monochloro-propylene glycol for the production of caffeine and guaiacol glycerol ether. Glycerol can participate in the cyclization and condensation of p-hydroxybenzaldehyde and, 4, 6-trihydroxy-3, 5-dimethyl benzophenone to obtain the expectorant cough medicine Rhododendron. Glycerol can be condensed with acetone to form 1, 2-isopropylidene glyceride. This can be used for the manufacture of shark liver alcohol used for increasing the count of white blood cell. Nitration of glycerol can generate glyceryl trinitrate, namely, vasodilator nitroglycerin. Glycerol can be reacted with 2, 5-diaminoanisole sulfate to give the intermediate 6-methoxy-4, 7-phenanthroline. Glycerol is also a raw material for the midrange marker 6-methoxy-7-nitroquinoline. Several quinoline derivatives were obtained from the reaction between glycerol and aromatic primary amines with such reactions being called skraup reactions. Another major application of glycerol is the preparation of alkyd resin. At present, the resin used in the paint around the world includes mainly alkyd resin, acrylic resin, vinyl resin and epoxy resin, among which, alkyd resin paint ranks first in both the United States and Japan. Glycerol accounts for 42% in the polyol used in the alkyd resin. Glycerol is easy to digest and non-toxic and can be used as a solvent, hygroscopic agent and vehicle for the food industry. For the seasoning and coloring food, because the glycerol is sticky, and can therefore contribute to food molding. During the rapid freezing of food, glycerol can be used as a direct medium of heat transfer for food. Glycerol is also a lubricant for food processing and packaging machinery. In addition, the application of polyglycerol and polyglycerol esters during the manufacture of crispy and margarine products is increasing year by year. Glycerol can be used in tobacco (mainly cigars) as a humectant to keep the moisture of the tobacco, to prevent embrittlement, and to increase the sweetness of tobacco. In the case of cigar paper and filter paper, it is used as a plasticizer in the form of triacetin. Glyceryl triacetate accounts for one third of the total consumption of glycerol in the tobacco industry. Between 1970 and 1986, the average annual growth rate of glycerol production in China was 5.3%, but the average annual growth rate of consumption in the same period was 7%. In 1983 – 1986, China imported a total of 52,400 tons of glycerol with the average annual import of 1.31 million tons, accounting for 1/4 of the annual consumption. Glycerol has been recognized as a non-toxic and safe substance with no harmful effect on human or animal upon oral administration of high-dose of natural glycerol. Intravenous injection of 5% glycerol solution to human also causes poisoning phenomenon. The national Institute of Occupational Safety and Health (NIOSH) provides that the glycerol content of water, when being higher than 1000mg/L, is harmless to the human body.
| [Preparation]
Glycerol(56-81-5) industrial production methods can be divided into two categories: method using natural oil as raw material with the resulting glycerol commonly known as natural glycerol; method using propylene as raw material with the resulting glycerol commonly known as synthetic glycerol. 1. Production of natural glycerol; before 1984, glycerol was all recovered from the by-products of soap manufacturing from grease of animals and plants. Until now, natural grease is still the main raw material for the production of glycerol with about 42% of the natural glycerol being made from by-products and 58% being made from fatty acids. Saponification of Oil in the soap Industry: The products in saponification reaction products are divided into two layers: the upper layer mainly contains fatty acid sodium salt (soap) and a small amount of glycerol, the lower layer is the waste lye, being the glycerol dilute solution containing salt and sodium hydroxide, generally containing 9-16% glycerol and 8-20% inorganic salt. Grease reaction: the glycerol water obtained from the grease hydrolysis (also known as sweet water) contains higher glycerol content than soap waste, being about 14-20% with 0-0.2% inorganic salt. In recent years, it has been widely applied of continuous high-pressure hydrolysis method. The reaction is free of catalyst and the obtained sweet water is generally free of inorganic acid, thus can be more easily purified than the waste lye. For both the soap waste liquid and the glycerol water obtained from oil hydrolysis, the glycerol is not high, and they contain all kinds of impurities. The production process of the natural glycerol includes purification, concentration to obtain crude glycerol, and refining process including crude glycerol distillation, decolorization and deodorization. This process is described in detail in some books. 2. The production of synthetic glycerol: those various pathways for glycerol synthesis from propylene can be summarized into two categories, namely chlorination and oxidation. Now the industry is still using propylene chlorination method and propylene non-periodic acetic acid oxidation method. (1) Propylene chlorination method: this is the most important production method of synthesizing glycerol, including a total of four steps, namely high-temperature propylene chlorination, chlorophenol hypochlorification, dichloropropanol saponification and epichlorohydrin hydrolysis. The production process of glycerol by epichlorohydrin hydrolysis is performed under 150 ° C and 1.37 MPa pressure of carbon dioxide in an aqueous solution of 10% hydroxide and 1% sodium carbonate. This can produce a glycerol aqueous solution containing 5-20% glycerol and sodium chloride, followed by concentration, desalting and distillation to obtain the glycerol with the purity of over 98%. (2) Method of propylene peracetic acid oxidation: propylene can interact with peracetic acid to generate propylene oxide with propylene oxide isomerization generating alkene to propanol. The latter reacts with peracetic acid to produce glycidyl alcohol (i.e. glycidyl), and finally hydrolyzed to glycerol. The production of peracetic acid does not require catalyst, acetaldehyde and oxygen gas phase oxidation. Under atmospheric pressure, 150-160 ℃ and the contact time of 24 s, the aldehyde has a conversion rate of 11% and the acetic acid has a selectivity of 83%. The latter two steps of the reaction can continuously proceeded in a special structure of the reaction distillation column. The raw material allyl alcohol and the ethyl acetate solution containing peracetic acid are sent to the column and the column is controlled at 60-70 ° C and 13-20 kPa. The top of the column can be evaporated of ethyl acetate solvent and water. At the tower kettle, we can obtain the glycerol aqueous solution. This method is selective and has high yield, taking peracetic acid as oxidant. It doesn’t need catalyst, and the reaction speed is high, simplifying the process. Production of 1t glycerol consumes 1.001 t allyl alcohol and 1.184 t peracetic acid with 0.947 t of acetic acid by-product. At present, both the production of natural glycerol and synthetic glycerol accounts of almost 50%. The propylene chlorination process accounts for about 80% of the total glycerol production. China's natural glycerol accounted for more than 90% of total output.
| [Category]
Flammable liquids | [Explosive hazardous characteristics]
It is explosive upon reaction with chromic anhydride, potassium chlorate and potassium permanganate. | [Acute toxicity]
oral-rat LD50: 26000 mg/kg; oral-mouse LD50: 4090 mg/kg
Stimulate Data Skin-Rabbit 500 mg/24 Hour Mild; Eyes-Rabbit 126 mg Mild | [Flammability and Hazardous characteristics]
It is combustible in case of fire, high temperature and strong oxidant with combustion releasing stimulating smoke | [Storage]
ventilated, low temperature and dry | [Fire extinguishing agent]
foam, dry powder, carbon dioxide, sand, mist water | [Occupational standard]
TWA 15 mg/m3 |
|
|