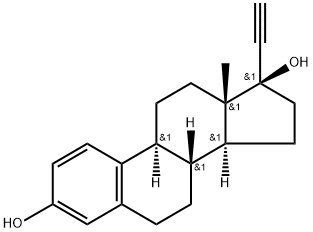
Ethinyl Estradiol
- CAS No.
- 57-63-6
- Chemical Name:
- Ethinyl Estradiol
- Synonyms
- ETHINYL ESTRADIOL;ETHYNYLESTRADIOL;17α-Ethynylestradiol;Estradio;17a-ethinylestradiol;17ALPHA-ETHYNYLESTRADIOL;(8R,9S,13S,14S,17R)-17-ethynyl-13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol;eed;17-Ethinylestradiol;LYNORAL
- CBNumber:
- CB1350377
- Molecular Formula:
- C20H24O2
- Molecular Weight:
- 296.41
- MDL Number:
- MFCD00003690
- MOL File:
- 57-63-6.mol
- MSDS File:
- SDS
| Melting point | 182-183 °C(lit.) |
|---|---|
| Boiling point | 378°C (rough estimate) |
| Density | 1.0944 (rough estimate) |
| refractive index | -30 ° (C=0.4, Pyridine) |
| Flash point | 9℃ |
| storage temp. | room temp |
| solubility | ethanol: 50 mg/mL, clear, slightly yellow |
| pka | pKa 10.32 (Uncertain) |
| color | White to Light yellow to Light orange |
| Merck | 14,3734 |
| BRN | 2419975 |
| BCS Class | 3/1 |
| InChIKey | BFPYWIDHMRZLRN-SLHNCBLASA-N |
| CAS DataBase Reference | 57-63-6(CAS DataBase Reference) |
| FDA UNII | 423D2T571U |
| NIST Chemistry Reference | Ethinyl estradiol(57-63-6) |
| Proposition 65 List | Ethinylestradiol |
| NCI Drug Dictionary | Estinyl |
| ATC code | G03CA01,L02AA03 |
| EPA Substance Registry System | Ethinyl estradiol (57-63-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H350-H410 | |||||||||
| Precautionary statements | P202-P264-P270-P273-P301+P312-P308+P313 | |||||||||
| Hazard Codes | T,F | |||||||||
| Risk Statements | 45-22-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 53-36/37/39-45-36/37-16 | |||||||||
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | RC8925000 | |||||||||
| F | 8 | |||||||||
| HS Code | 29372390 | |||||||||
| Toxicity | LD50 oral in rat: 960mg/kg | |||||||||
| NFPA 704 |
|
Ethinyl Estradiol price More Price(38)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | E-076 | 17α-Ethynylestradiol solution 1.0?mg/mL in methanol, ampule of 1?mL, certified reference material, Cerilliant? | 57-63-6 | 1mL | $131 | 2024-03-01 | Buy |
| Sigma-Aldrich | BP421 | Ethinylestradiol British Pharmacopoeia (BP) Assay Standard | 57-63-6 | 100MG | $257 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1260001 | Ethinyl estradiol United States Pharmacopeia (USP) Reference Standard | 57-63-6 | 150mg | $348.8 | 2024-03-01 | Buy |
| TCI Chemical | E0037 | Ethynylestradiol >98.0%(HPLC)(T) | 57-63-6 | 1g | $71 | 2024-03-01 | Buy |
| TCI Chemical | E0037 | Ethynylestradiol | 57-63-6 | 5G | $213 | 2024-03-01 | Buy |
Ethinyl Estradiol Chemical Properties,Uses,Production
Description
Ethynyl estradiol (EE) is a synthetic form of estrogen that is majorly employed in numerous hormonal contraceptives in combination with progestins. Occasionally, it is also used as a constituent of menopausal hormone therapy with combination with progestins for treating menopausal symptoms. Previously, the drug was used solely for numerous indications such as treatment of prostate cancer and gynecological disorders.
Ethynyl estradiol is a semisynthetic alkylated estradiol with a 17-alpha-ethinyl substitution. It is normally administred orally and is marketed mostly as a combination oral contraceptive under various brand names such as Alesse, Tri-cyclen, Yasmin, and Triphasil. A black box warning is normally labelled on the packaging that states that the drug should not be used in smoking women who are over 35 years old as it can lead to increased risk of serious cardiovascular side effects.
History
Ethynyl estradiol was first developed in 1930 and officially introduced for medical used in 1943. In the 1960s, the drug was widely used in birth control pills. EE is currently found in most combined forms of birth control pills, which makes it one of the most used estrogens.
Indications
Ethynyl estradiol is used for treatment of moderate to severe vasomotor symptoms such as night sweats, hot flashes, and flushing that are associated with the menopause, prostatic carcinoma-palliative therapy of advanced disease, female hypogonadism, as emergency contraceptive, breast cancer, and as an oral contraceptive.
Pharmacodynamics
Ethynyl estradiol is a synthetic derivative of the natural estrogen estradiol. The drug is one of two widely used in oral contraceptive pills. Mestranol is the other drug that is normally converted to ethinyl estradiol before it becomes biologically active. EE is used together with norethindrone as an oral contraceptive agent.
Mechanism of Action
The estrogen in the drug diffuse into their target cells before interacting with a protein receptor. Notably, target cells include the female reproductive tract, the hypothalamus, the mammary gland, and the pituitary. Estrogens act by increasing the hepatic synthesis of thyroid binding globulin (TBG), sex hormone binding globulin (SHBG) as well as other serum proteins. It also acts by suppressing follicle-stimulating hormone (FSH) from anterior pituitary. All these activities are initiated by fist binding to the estrogen receptors. The hypothalamic-pituitary system is suppressed by the combination of an estrogen with a progestin, which decreases the secretion of gonadorotropin-releasing hormone (GnRH).
Medical Uses
Ethinyl Estradiol is majorly used to control pregnancy after sex as a contraception in combined oral contraceptives (COC), which are also referred to as birth control. EE is not only used in preventing pregnancy but is also employed in the treatment of absence of menstruation, acne, as well as symptoms during menstruation. It majorly works by preventing ovulation (the release of an egg) during the menstrual cycle. Further, it acts by making the vaginal fluid thicker to help in preventing sperm from fertilizing the released egg. It also changes the lining of the uterus to prevent attachment of a fertilized egg. EE also works by making the menstruation cycle more regular, decreasing blood loss and painful periods, and decreasing the risk of ovarian cysts.EE is also used as menopausal hormone therapy (HRT) which has numerous benefits such as vaginal itching, vaginal dryness (which normally causes pain during sexual intercourse), and depressed mood. Previously, it was used as a constituent of feminizing hormone therapy for transgender women; however, the use of estradiol has largely superseded it in this therapy. The drug can also be used in preventing osteoporosis and in treating hypogonadism in women and has been used as palliative care for breast cancer in women and prostate cancer in men.
Contraindications
Ethnynyl estradiol should not be prescribed to individuals with history of susceptibility to venous or arterial thrombosis (blood clots) as it can lead to increased cardiovascular problems such as myocardial infarction, venous thromboembolism, and ischemic stroke. As such, it women with acute deep vein thrombosis or pulmonary embolism, any vascular disease history of DVT/PE, and complicated valvular heart disease are not advised to take the drug.
Side Effects
The side effects of EE include vomiting, nausea, headache, tenderness of the breast, abdominal cramps/bloating, swelling of the feet/ankles, and change in weight. In case of severe effects, one should immediately contact the doctor. EE can also cause irregular periods, which is considered normal.
Precautions
Before taking EE, it is important to inform the doctor or pharmacist if you allergic to the drug or to any other estrogens such as mestranol. It is also important to inform the doctor if one has a medical history of blood clots, for instance in the eyes, legs, and lungs. The medication can also affect blood sugar levels if one is diabetic. However, it is important to monitor the blood sugar levels and share it with the doctor in case one is using the drug.
Interactions
Drug interactions may change how EE works or might increase its side effects. Products that can interact with the drug include aromatase inhibitors (such as exemestane and anastrozole), tizanidine, tamoxifen, and tranexamic. It can also interact with certain combination products that are normally used to treat chronic hepatitis C. some drugs interact with EE by decreasing the amount of the drug in the body.
Description
Estrogens direct the development of the female genotype in embryogenesis and at puberty. Estradiol is the major estrogen secreted by the premenopausal ovary. Ethynyl estradiol is a synthetic analog of 17β-estradiol . A USP-approved grade of ethynyl estradiol is often formulated in combination with a progestin such as norgestrel /levonorgestrel or desogestrel and provided for use as an oral contraceptive. Efficacy of oral administration of ethynyl estradiol is facilitated by the ethynyl substitution at the C-17 position, which inhibits first pass hepatic metabolism. Ethynyl estradiol is also rapidly and almost completely absorbed from the gastrointestinal tract.
Chemical Properties
Off-White to Light-Yellow Crystalline Powder
Chemical Properties
Estradiol, 17-β-is an odorless white to yellow crystalline substance.
Chemical Properties
Ethinylestradiol is a white to creamy-white powder. Odorless.
Originator
Estinyl,Schering,US,1944
Uses
estrogen, plus progestogen as oral contraceptive
Uses
A synthetic estradiol analog.
Uses
A synthetic steroid with high oral estrogenic potency
Uses
A metabolite of 17a-Ethynylestradiol
Definition
ChEBI: A 3-hydroxy steroid that is estradiol substituted by a ethynyl group at position 17. It is a xenoestrogen synthesized from estradiol and has been shown to exhibit high estrogenic potency on oral administration.
Manufacturing Process
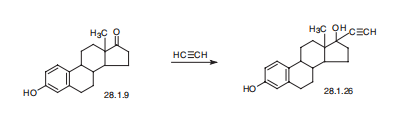
In about 250 cc of liquid ammonia (cooled with dry ice and acetone) are dissolved about 7.5 g of potassium and into the solution acetylene is passed until the blue color has disappeared (about 3 hours). Then slowly a solution or suspension of 3 g of estrone in 150 cc of benzene and 50 cc of ether is added. The freezing mixture is removed, the whole allowed to stand for about 2 hours and the solution further stirred overnight. Thereupon the reaction solution is treated with ice and water, acidified with sulfuric acid to an acid reaction to Congo red and the solution extracted five times with ether. The combined ether extracts are washed twice with water, once with 5% sodium carbonate solution and again with water until the washing water is neutral. Then the ether is evaporated, the residue dissolved in a little methanol and diluted with water. The separated product is recrystallized from aqueous methanol. The yield amounts to 2.77 g. The 17-ethinyl-estradiol-3,17 thus obtained melts at 142°C to 144°C.
brand name
Estinyl (Schering); Feminone (Pharmacia & Upjohn); Lynoral (Organon).
Therapeutic Function
Estrogen
General Description
17 -Ethinyl estradiol has thegreatest advantage over other estradiol products of beingorally active. It is equal to estradiol in potency by injectionbut is 15 to 20 times more orally active. The primary metabolicpath for ethinyl estradiol is 2-hydroxylation bycytochrome P450 isozyme 3A4 (CYP3A4), followed byconversion to the 2- and 3-methyl ethers by catechol-Omethyltransferase.The 3-methyl ether of ethinyl estradiolis mestranol, USP, used in oral contraceptives. Mestranolis a prodrug that is 3-O-demethylated to the active ethinylestradiol. An oral dose of about 50 μg of mestranol has anestrogenic action approximately equivalent to 35 g oforal ethinyl estradiol. The demethylation is mainly mediatedby CYP2C9.
General Description
Fine white to creamy white powder. A synthetic steroid. Used in combination with progestogen as an oral contraceptive.
Air & Water Reactions
Air and light sensitive . Insoluble in water.
Reactivity Profile
Ethynyl estradiol may react vigorously with strong oxidizing agents. May react exothermically with reducing agents to generate gaseous hydrogen.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition Ethynyl estradiol emits acrid smoke and fumes.
Fire Hazard
The flash point data for Ethynyl estradiol are not available. Ethynyl estradiol is probably combustible.
Biochem/physiol Actions
17α-Ethynylestradiol is an orally bio-active synthetic estrogen used as an oral contraceptive.
Mechanism of action
Synthetic estrogen with potent activity (inhibition of ovulation), widely used in oral contraceptives. Manufactured from natural estrogen, estrone, by reaction with potassium acetylide (HCRCK) in liquid ammonia. The synthetic 17α-ethynyl derivative of estradiol-17β. The 17α-ethynyl group increases the in vivo potency of estradiol- 17β by blocking the action of 17β-dehydrogenase, a major pathway of estradiol-17β metabolic inactivation. It is thus active orally and is among the most potent of the known estrogenic compounds.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, tumorigenic, and neoplastigenic data. Poison by intraperitoneal route. Moderately toxic by ingestion. Human systemic effects by ingestion: glandular effects. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTRADIOL
Synthesis
Ethinyl estradiol, 17|á-ethinyl-1,3,5(10)-estratrien-3-17|?-diol (28.1.26), is made either by condensing estrone with acetylene in the presence of potassium hydroxide (Favorskii reaction), or by reacting sodium acetylenide in liquid ammonia with estrone.
Potential Exposure
The working environment may be contaminated during sex hormone manufacture, especially during the extraction and purification of natural steroid hormones; grinding of raw materials; handling of powdered products and recrystallization. Airborne particles of sex hormones may be absorbed through the skin, ingested or inhaled. Enteric absorption results in quick inactivation of sex hormones in the liver. The rate of inactivation is decreased for the oral, alkylated steroid hormones (methyl testosterone, anabolic steroids, etc.). Sex hormones may accumulate and reach relatively high levels even if their absorption is intermittent. Consequently, repeated absorption of small amounts may be detrimental to health. Intoxication by sex hormones may occur in almost all the exposed workers if preventive measures are not taken. The effect in the industrial sector is more successful than the agricultural one (chemical caponizing of cockerels by stilbestrol implants and incorporation of estrogens in feed for body weight gain promotion in beef cattle), where measures taken are summary and the number of cases of intoxication is consequently bigger
First aid
Skin Contact: Flood all areas of body thathave contacted the substance with water. Do not wait toremove contaminated clothing; do it under the water stream.Use soap to help assure removal. Isolate contaminatedclothing when removed to prevent contact by others. EyeContact: Remove any contact lenses at once. Immediatelyflush eyes well with copious quantities of water or normalsaline for at least 20-30 min. Seek medical attention.Inhalation: Leave contaminated area immediately; breathefresh air. Proper respiratory protection must be supplied toany rescuers. If coughing, difficult breathing, or any othersymptoms develop, seek medical attention at once, even ifsymptoms develop many hours after exposure. Ingestion:Contact a physician, hospital, or poison center at once. Ifthe victim is unconscious or convulsing, do not inducevomiting or give anything by mouth. Assure that thepatient’s airway is open and lay him on his side with hishead lower than his body and transport immediately to a medical facility. If conscious and not convulsing, give aglass of water to dilute the substance. Vomiting should notbe induced without a physician’s advice.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in a refrigerator under an inert atmosphereand protect from exposure to light.
Shipping
UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
17--Ethynylestradiol forms a hemihydrate on recrystallising from MeOH/H2O. It dehydrates on melting and remelts on further heating at m 182-184o. The UV has max at 281nm ( 2040) in EtOH. Its solubility is 17% in EtOH, 25% in Et2O, 20% in Me2CO, 25% in dioxane and 5% in CHCl3. [Petit & Muller Bull Soc Chim Fr 121 1951.] The diacetyl derivative has m 143-144o (from MeOH) and [] D 20 +1o (c 1, CHCl3) [Mills et al. J Am Chem Soc 80 6118 1958]. [Beilstein 6 IV 6877.]
Incompatibilities
May react exothermically with reducing agents to generate flammable gaseous hydrogen. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides.
Waste Disposal
It is inappropriate and possibly dangerous to the environment to dispose of expired or waste drugs and pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator
Ethinyl Estradiol Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Wuhan Han Sheng New Material Technology Co.,Ltd | +8617798174412 | admin01@hsnm.com.cn | China | 2118 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@airuikechemical.com | China | 994 | 58 |
| Dorne Chemical Technology co. LTD | +86-13583358881 +86-18560316533 | Ethan@dornechem.com | China | 294 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Wuhan Haorong Biotechnology Co.,ltd | +8618565342920 | sales@chembj.net | China | 269 | 58 |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 | admin@guyunchem.com | China | 616 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7845 | 58 |
| Shandong Hanjiang Chemical Co., Ltd | +86-0533-2066820 +8618369939125 | hanson@sdhanjiang.com | China | 1527 | 58 |
| Wuhan Xinhao Biotechnology Co., Ltd | +86-18120578002 +86-18120578002 | xinhao-6@xinhaoshengwu.com | China | 350 | 58 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
Related articles
- Levonorgestrel-Ethinyl Estradiol Oral: uses and side effects
- This combination hormone medication Levonorgestrel-Ethinyl Estradiol is used to prevent pregnancy.
- Mar 20,2024
View Lastest Price from Ethinyl Estradiol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | Ethynyl estradiol
57-63-6
|
US $1.00 / g | 1g | 99% | 100kg | Dorne Chemical Technology co. LTD | |
 |
2024-04-26 | Ethynyl estradiol
57-63-6
|
US $0.90 / g | 10g | 99% | 300tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-24 | Ethynyl estradiol
57-63-6
|
US $0.00-0.00 / KG | 1KG | 99% | 500 | Wuhan Haorong Biotechnology Co.,Ltd |
-

- Ethynyl estradiol
57-63-6
- US $1.00 / g
- 99%
- Dorne Chemical Technology co. LTD
-

- Ethynyl estradiol
57-63-6
- US $0.90 / g
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Ethynyl estradiol
57-63-6
- US $0.00-0.00 / KG
- 99%
- Wuhan Haorong Biotechnology Co.,Ltd
57-63-6(Ethinyl Estradiol)Related Search:
1of4