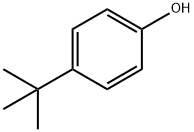
4-tert-Butylphenol
- CAS No.
- 98-54-4
- Chemical Name:
- 4-tert-Butylphenol
- Synonyms
- PTBP;P-TERT-BUTYLPHENOL;PARA-TERT-BUTYLPHENOL;4-(1,1-DIMETHYLETHYL)PHENOL;t-Butylphenol;p-terc.Butylfenol;Phenol, 4-tert-butyl-;4-tert-Butylphenol, extra pure;BUTYLPHEN;FEMA 3918
- CBNumber:
- CB1854749
- Molecular Formula:
- C10H14O
- Molecular Weight:
- 150.22
- MDL Number:
- MFCD00002367
- MOL File:
- 98-54-4.mol
- MSDS File:
- SDS
| Melting point | 96-101 °C (lit.) |
|---|---|
| Boiling point | 236-238 °C (lit.) |
| Density | 0.908 g/mL at 25 °C (lit.) |
| vapor pressure | 1 mm Hg ( 70 °C) |
| refractive index | 1.4787 |
| FEMA | 3918 | P-TERT-BUTYLPHENOL |
| Flash point | 113 °C |
| storage temp. | Store below +30°C. |
| solubility | ethanol: soluble50mg/mL, clear, colorless |
| form | Flakes or Pastilles |
| pka | 10.23(at 25℃) |
| color | White to light beige |
| Specific Gravity | 0.908 |
| PH | 7 (10g/l, H2O, 20℃) |
| Odor | at 1.00 % in propylene glycol. oakmoss leather |
| Odor Type | leathery |
| explosive limit | 0.8-5.3%(V) |
| Water Solubility | 8.7 g/L (20 ºC) |
| JECFA Number | 733 |
| Merck | 14,1585 |
| BRN | 1817334 |
| Stability | Stable. Incompatible with copper, steel, bases, acid chlorides, acid anhydrides, oxidizing agents. |
| InChIKey | QHPQWRBYOIRBIT-UHFFFAOYSA-N |
| LogP | 3 at 23℃ |
| FDA 21 CFR | 328.10; 175.300 |
| Substances Added to Food (formerly EAFUS) | 4-(1,1-DIMETHYLETHYL)PHENOL |
| CAS DataBase Reference | 98-54-4(CAS DataBase Reference) |
| FDA UNII | O81VMW36CV |
| NIST Chemistry Reference | Phenol, p-tert-butyl-(98-54-4) |
| EPA Substance Registry System | p-tert-Butylphenol (98-54-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H315-H318-H361f-H410 | |||||||||
| Precautionary statements | P202-P273-P280-P302+P352-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | Xi,N | |||||||||
| Risk Statements | 37/38-41-51/53-62-38-37-34 | |||||||||
| Safety Statements | 26-39-61-36/37/39-45 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | SJ8925000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29071900 | |||||||||
| Toxicity | LD50 orally in rats: 3.25 ml/kg (Smyth) | |||||||||
| NFPA 704 |
|
4-tert-Butylphenol price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | B99901 | 4-tert-Butylphenol 99% | 98-54-4 | 1kg | $118 | 2024-03-01 | Buy |
| Sigma-Aldrich | 506761 | 4-tert-Butylphenol PESTANAL?, analytical standard | 98-54-4 | 1000mg | $26.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 425761 | 4-tert-Butylphenol purified by sublimation, 99% | 98-54-4 | 5g | $40.8 | 2023-06-20 | Buy |
| TCI Chemical | B0230 | 4-tert-Butylphenol >98.0%(GC) | 98-54-4 | 25g | $17 | 2024-03-01 | Buy |
| TCI Chemical | B0230 | 4-tert-Butylphenol >98.0%(GC) | 98-54-4 | 500g | $27 | 2024-03-01 | Buy |
4-tert-Butylphenol Chemical Properties,Uses,Production
Description
Para-tertiary-butylphenol formaldehyde resin (PTBPF- R) is a polycondensate of para-tertiary-butylphenol and formaldehyde. Major occupational sources are neoprene glues and adhesives in industry, in the shoemaking and leather industry or in car production. It is also used as a box preservative in box and furniture manufacture, and in the production of casting moulds, car-brake linings, insulated electrical cables, adhesives, printing inks and paper laminates. Para-tertiary-butyl-phenol is the sensitizer.
Chemical Properties
4-tert-Butylphenol is a white to pale yellow crystalline solid at room temperature and is sold in solid form as flakes or briquettes. 4-tert-butylphenol is employed in coating products, polymers, adhesives, sealants and for the synthesis of other substances.
The major use is as a monomer in chemical synthesis, e.g. for the production of polycarbonate, phenolic resins, epoxy resins. PtBP is used as a chain terminator in the synthesis of polycarbonate polymers. The main uses of polycarbonate are in compact discs, DVD and CD Rom manufacture.
Occurrence
Reported found in origanum (Coridothymus cap. (L.) Richb.)
Uses
Polycarbonate Chain Terminator, Glycidyl Ethers; Phosphate Esters, Fragrances, Oil Field Chemicals-Demulsifiers; Plasticizer for cellulose acetate; intermediate for antioxidants, special starches, oil-soluble phenolic resins; pour-point depressors and emulsion breakers for petroleum oils and some plastics; synthetic lubricants; insecticides; industrial odorants; motor-oil additives.
Uses
4-tert-butylphenol on condensation with formaldehyde gives calix[5]arene which is used in enzyme mimetics.
Uses
4-tert-Butylphenol is a phenol derivative. Its contact with skin may lead to leukoderma. It is widely used in the polymer industry. Reaction of 4-tert-Butylphenol with mushroom tyrosinase has been reported to afford 4-t-butyl-o-benzoquinone and kinetics of this enzymatic reaction has been investigated.
Definition
ChEBI: 4-tert-butylphenol is a member of the class of phenols that is phenol substituted with a tert-butyl group at position 4. It has a role as an allergen.
Application
4-tert-Butylphenol may be employed as carbon and energy supplement in the culture medium of Sphingobium fuliginis strains.
4-tert-Butylphenol is suitable reagent used in kinetic study of hydroxylation of 4-tert-butylphenol by mushroom tyrosinase. It may be used in the synthesis of calix[7]arene.
Preparation
Prepared by heating phenol with isobutanol in the presence of zinc chloride; also from phenol, tert- butyl chloride and excess alkali in alcohol
Synthesis Reference(s)
The Journal of Organic Chemistry, 22, p. 988, 1957 DOI: 10.1021/jo01359a609
General Description
Crystals or practically white flakes. Has a disinfectant-like odor. May float or sink in water. Insoluble in water.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Phenols, such as 4-tert-Butylphenol, do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid.
Hazard
Irritant to eyes and skin.
Fire Hazard
Combustible.
Contact allergens
Para-tert-butylphenol is used with formaldehyde to produce the polycondensate p-tert-butylphenol-formaldehyde resins (PTBPFR). Major occupational sources are neoprene glues and adhesives in industry, in the shoemaking and leather industries or in car production. It is also used as a box preservative in box and furniture manufacture and in the production of casting molds, car brake linings, insulated electrical cables, adhesives, printing inks, and paper laminates. Para-tertbutylphenol seems to be the sensitizer
Safety Profile
Poison by intraperitoneal route. Moderately toxic by skin contact and ingestion. A skin and severe eye irritant. Questionable carcinogen with experimental neoplastigenic data. Combustible when exposed to heat or flame; can react with oxidizing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid and irritating fumes. See also PHENOL and other butyl phenols.
Potential Exposure
Butylphenols may be used as intermediates in manufacturing varnish and lacquer resins; as a germicidal agent in detergent disinfectants; as a pour point depressant, in motor-oil additives; de-emulsifier for oil; soap-antioxidant, plasticizer, fumigant, and insecticide
Shipping
UN2430 Alkylphenols, solid, n.o.s. (including C2-C12 homologues), Hazard class: 8; Labels: 8— Corrosive material
Purification Methods
Crystallise the phenol to constant melting point from pet ether (b 60-80o). It sublimes in vacuo. Also purify it via the benzoate, as for phenol. The salicylate ester [87-18-30] has m 63-64o (from aqueous EtOH, or EtOH). [Beilstein 6 IV 3296.]
Incompatibilities
Vapors may form explosive mixture with air. These phenol/cresol materials can react with oxidizers; reaction may be violent. Incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may cause the gas to ignite and explode. Heat is also generated by the acid-base reaction with bases; such heating may initiate polymerization of the organic compound. React with boranes, alkalies, aliphatic amines, amides, nitric acid, sulfuric acid. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). These reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid and can explode when heated. Many phenols form metal salts that may be detonated by mild shock
4-tert-Butylphenol Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| NINGBO INNO PHARMCHEM CO., LTD. | 13867897135 | sales@nbinno.com | CHINA | 925 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
View Lastest Price from 4-tert-Butylphenol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-06-30 | 4-tert-Butylphenol
98-54-4
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2022-09-27 | 4-tert-Butylphenol
98-54-4
|
US $20.00 / kg | 1kg | 99% | 5000 Ton | Hebei Duling International Trade Co. LTD | |
 |
2021-07-02 | 4-tert-Butylphenol
98-54-4
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- 4-tert-Butylphenol
98-54-4
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- 4-tert-Butylphenol
98-54-4
- US $20.00 / kg
- 99%
- Hebei Duling International Trade Co. LTD
-

- 4-tert-Butylphenol
98-54-4
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd