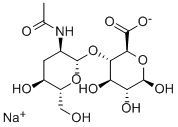
Sodium hyaluronate
- CAS No.
- 9067-32-7
- Chemical Name:
- Sodium hyaluronate
- Synonyms
- hyaluronic;Hyaluronate;HYALURONIC ACID SODIUM;Sph;HYALURONIC ACID SODIUM SALT;Hyaluronesodium;Sodium Hyaluronic;3000 Da;HYALURONIC ACID NA-SALT;sodium hyaluronate powder
- CBNumber:
- CB4176691
- Molecular Formula:
- C14H22NNaO11
- Molecular Weight:
- 403.31
- MDL Number:
- MFCD00875848
- MOL File:
- 9067-32-7.mol
- MSDS File:
- SDS
| Melting point | >209°C (dec.) |
|---|---|
| alpha | D25 -74° (c = 0.25 in water): Rapport et al., J. Am. Chem. Soc. 73, 2416 (1951) |
| storage temp. | -20°C |
| solubility | H2O: 5 mg/mL, clear, colorless |
| form | Powder |
| color | White to cream |
| PH | pH(2g/l,25℃) : 5.5~7.5 |
| Water Solubility | SOLUBLE |
| Merck | 13,4776 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | MAKUBRYLFHZREJ-JWBQXVCJSA-M |
| SMILES | [C@@H]1(O[C@H]2[C@H](O)[C@H]([C@H](O)O[C@@H]2C(=O)[O-])O)O[C@H](CO)[C@@H](O)C[C@H]1NC(=O)C.[Na+] |&1:0,2,3,5,6,9,15,18,21,r| |
| LogP | -6.623 (est) |
| CAS DataBase Reference | 9067-32-7(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | YSE9PPT4TH |
SAFETY
Risk and Safety Statements
| Hazard Codes | B,Xi | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 22-24/25-36/37/39-27-26 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | MT7250000 | |||||||||
| F | 3-10 | |||||||||
| HS Code | 39139000 | |||||||||
| Toxicity | LD50 oral in rabbit: > 1gm/kg | |||||||||
| NFPA 704 |
|
Sodium hyaluronate price More Price(140)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 02737 | Hyaluronic acid sodium salt from Streptococcus equi mol wt 1,750,000-2,000,000 | 9067-32-7 | 10mg | $145.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 02737 | Hyaluronic acid sodium salt from Streptococcus equi mol wt 1,750,000-2,000,000 | 9067-32-7 | 50mg | $800 | 2024-03-01 | Buy |
| Sigma-Aldrich | 00941 | Hyaluronic acid sodium salt from Streptococcus equi mol wt 300,000-500,000 | 9067-32-7 | 10MG | $508 | 2023-06-20 | Buy |
| Sigma-Aldrich | 00941 | Hyaluronic acid sodium salt from Streptococcus equi mol wt 300,000-500,000 | 9067-32-7 | 50MG | $1990 | 2023-06-20 | Buy |
| Alfa Aesar | J66993 | Hyaluronic acid sodium salt, Streptococcus equi, 91% | 9067-32-7 | 500mg | $54.5 | 2024-03-01 | Buy |
Sodium hyaluronate Chemical Properties,Uses,Production
Physicochemical property
Sodium hyaluronate is also called Hyaluronic acid sodium, is physiological active substances, which is widely exists in the human body. It is high molecular weignt straight-chain mucopolysaccharides, which is composed of glucuronic acid and acetyl amino hexose polymerizatio. its molecular weight is 1 million. In water, It can form a thick viscoelastic solution, has the physiological ph and ionic strength. The molecular configuration is variable, so also can be done with a fine needle. Sodium hyaluronate won't produce inflammatory substances , called Healon. The 10 mg dissolved in 1 ml saline solution, its thick viscosity is 200000 times higher than aqueous humor or saline water. Healon protein content is less than 0.5%, and a sterile solution with high purity.
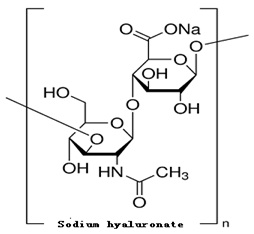
Figure 1 The structure of Sodium hyaluronate
Sodium Hyaluronate Injection
Hyaluronic acid sodium is a class of the non-drug neutral medium. Sodium hyaluronate injection is colorless transparent viscous liquid. Sodium hyaluronate is the main component of synovial joints , one of the components of cartilage matrix,and plays a role of lubrication in articular cavity, can reduce friction between organization, can play a role of the elastic at the same time and act the buffer effect of stress on articular cartilage, has their physiological function. High molecular weight, high concentration and high polymer viscoelasticity of sodium hyaluronate can significantly improve the joint inflammatory reaction, strengthen the function of joint fluid sticky and lubrication, protect the articular cartilage, promote the healing and regeneration of the articular cartilage, relieve pain, and increase the motion of Joint. The common dosage of sodium hyaluronate is: the jaw joints with 1 ml (10 mg), shoulder joint in 2 ml (20 mg), hip and knee with 2.5 ml (25 mg). It is mainly used in the treatment of degenerative osteoarthritis, traumatic arthritis, chronic rheumatic or rheumatoid arthritis, temporal jaw arthritis, femoral head aseptic necrosis.
Application in Particular Diseases
In Osteoarthritis:
- Four intraarticular hyaluronic acid preparations are available for treating pain associated with OA of the knee: sodium hyaluronate (Hyalgan 20 mg/2 mL; Supartz 25 mg/2.5 mL), hylan polymers (Synvisc 16 mg/2 mL), and hyaluronan (Orthovisc 30 mg/2 mL). Hyalgan and Supartz are administered once weekly for five injections, whereas Synvisc and Orthovisc are administered once weekly for three injections.
- Injections are well tolerated, but acute joint swelling and local skin reactions (e.g., rash, ecchymoses, or pruritus) have been reported.
- These products may be beneficial for OA of the knee that is unresponsive to other therapy, but they are expensive because treatment includes both drug and administration costs.
Pharmacological action
Sodium hyaluronate has the effect of promotinig wound healing. Sodium hyaluronate can form a macromolecular network barrier on the surface of eye tissue, preventing inflammatory content into the wound and lesion area, and can obviously increase the viscosity of liquids and adhesion, make the drug reserved and concentrated on the surface of the cornea for a long time, achieving long-term, powerful function. Sodium hyaluronate can stabilize tear film, prolong tear film broken, alleviate symptoms of dry eye. It is used for keratitis, corneal ulcer, the cornea injury caused by physical factors or other chemical burns.
Pharmacodynamics
(1)It can protect corneal endothelial in surgery, epithelium, iris and retina.
(2)It can be instead of aqueous humor without loss, so it can keep the depth of anterior chamber during the surgery.
(3)It is injected into glass cavity ,helping to reset the retina because of oppression.
(4)It can enter the conjunctiva under the pillow to keep filter pillow uplift in glaucoma surgery.
(5) It can help to reduce the adhesion for separation tissue surface.
Pharmacokinetics
Sodium hyaluronate is a component of the aqueous humor and vitreous body. When injected into anterior chamber or vitreous cavity, it can make a temporary increase in the concentration in every parts. In the anterior chamber, Healon gradually dilute by newborn aqueous humor and is discharged through the corner sclera sinus; Injected Healon into the vitreous cavity and again dilute aqueous humor then seep out . The monkey eyes experiment shows that Healon disappear entirely after 60~70 d in vitreous cavity. In the process, the concentration of Healon gradually weakened and its molecular remains the same. Healon injection into Monkey’s anterior chamber disappeared within 1 weeks. Clinical observation is similar to that of human eye.
Clinical application
Used in the surgeries such as intraocular lens, cataracts, glaucoma, corneal transplantation and retinal detachment .
Usage dosage
The injection is used commonly in anterior chamber, and each 0.5~0.5 ml.
In Intraocular lens implant surgery, using Healon can keep up the entire operation process of the depth of anterior chamber, having wide position for the operation of cataract extraction and intraocular lens implantation, also can keep the shape and position of the tissue in anterior chamber to run the operation smoothly and reduce early post-operational complications. Healon which is in corneal transplant applicationcan, can protect the corneal endothelium from the tissue damage.
Adverse reaction
A minority of patients using Healon may be a transient (25~30 MMHG) elevated intraocular pressurewith after operation,then back to normal in 1~2 d. But at the end of the surgery injection balance the brine into anterior chamber to dilute Healon, did not have the phenomenon of elevated intraocular pressure.
Precautions
1.Topical (eye drop) can sometimes have a thrill, foreign body sensation, itching, reddening, diffuse surface keratitis, etc. After eye surgery, this drug with blood or residual crystals mixing in the eye , can delay absorption and inflammation, and short elevated intraocular pressure. Local injection can have a sexual pain swelling or fever. Possible allergies are: sometimes may be blepharitis, eyelid skin inflammation, individual patients can also be a skin rash, itching, rare shock, urticaria, etc.
2. [contraindications]Allergies to this medicine , venous and lymphatic return leg disorders, patients with knee infection or inflammation, Articular cavity infection of acute phase to disable the articular cavity injection. To diabetic patients with no crystal, tis ban to the use of this drug in large quantities in surgery.
3. Caution:Allergies to the drugs, liver dysfunction or a history of liver function obstacle, the elderly, children, pregnancy, nursing mothers.
4. The use of this drug in the operation should be avoiding excessive packing. After the surgery with a balanced salt solution according to the need to remove residual liquid, this can control the intraocular pressure in case of elevated intraocular pressure.
5. When used in osseous arthritis, It can be combined with prednisone dragon. This can relieve pain, and recover joint function.
6. When it used in eye surgery, we should be closely monitoring intraoperative and postoperative intraocular pressure.
About the information about the physical and chemical properties pharmacological action, pharmacokinetics, clinical application usage and dosage of sodium hyaluronate in ophthalmology by chemcialbook editor yaoyao(2015-09-24).
Chemical Properties
The PhEur 6.3 describes sodium hyaluronate as the sodium salt of hyaluronic acid, a glycosaminoglycan consisting of D-glucuronic acid and N-acetyl-D-glucosamine disaccharide units. Sodium hyaluronate occurs as white to off-white powder or granules. It is very hygroscopic.
Uses
sodium hyaluronate is used as a humectant to increase moisture in the skin, it may also serve as an emulsifier. Sodium hyaluronate is capable of binding 1,800 times its own weight in water. It is the sodium salt of hyaluronic acid and is the most commonly used form.
Application
Sodium Hyaluronic is is an anionic, nonsulfated glycosaminoglycan distributed widely throughout connective, epithelial, and neural tissues. Sodium hyaluronate is widely used as follows:
antifungal agent.
prevents thromboembolic complications.
aldosterone antagonist used as an adjunct in the management of chronic heart failure.
suitable as substrate for hyaluronidase.
Surgical aid (ophthalmological).
Definition
Sodium Hyaluronate is a polysaccharide of linear and repeating structural units of the disaccharide D-glucuronic acid and N-acetylD-glucosamine. Hyaluronic acid in human body usually present as polymers with several million molecular weight, and the structural difference by the animal species has not been confirmed.
Production Methods
Sodium hyaluronate occurs naturally in vitreous humor, serum, chicken combs, shark skin, and whale cartilage; it is usually extracted and purified from chicken combs. It may also be manufactured by fermentation of selected Streptococcus zooepidemicus bacterial strains; sodium hyaluronate is removed from the fermentation medium by filtration and purified by ultrafiltration. It is then precipitated with an organic solvent and dried.
brand name
Equron [Veterinary] (Fort Dodge Animal Health); Legend (Bayer Animal Health); Synacid [Veterinary] (Schering-Plough Animal Health).
Pharmaceutical Applications
Sodium hyaluronate is the predominant form of hyaluronic acid at
physiological pH. The name hyaluronan is used when the
polysaccharide is mentioned in general terms, and in the literature
the terms hyaluronic acid and sodium hyaluronate are used
interchangeably.
Hyaluronan is used therapeutically to treat osteoarthritis in the
knee, and is an effective treatment for arthritic pain. Crosslinked
hyaluronan gels are used as drug delivery systems.
Hyaluronan is the most common negatively charged glycosaminoglycan
in the human vitreous humor, and is known to interact
with polymeric and liposomal DNA complexes, where hyaluronan
solutions have been shown to decrease the cellular uptake of
complexes.(4) This is useful for enhancing the availability and
retention time of drugs administered to the eye. It is immunoneutral,
which makes it useful for the attachment of biomaterials for use in
tissue engineering and drug delivery systems; it also has important
applications in the fields of vascosurgery and vascosupplementation.
Biochem/physiol Actions
High molecular mass polymer composed of repeating dimeric units of glucuronic acid and N-acetyl glucosamine which forms the core of complex proteoglycan aggregates found in extracellular matrix.
Safety
Sodium hyaluronate is used in cosmetics and in topical, parenteral,
and ophthalmic pharmaceutical formulations. It is generally
regarded as a relatively nontoxic and nonirritant material. Sodium
hyaluronate has been reported to be an experimental teratogen.
LD50 (mouse, IP): 1.5 g/kg
LD50 (rabbit, IP): 1.82 g/kg
LD50 (rat, IP): 1.77 g/kg
storage
Sodium hyaluronate should be stored in a cool, dry place in tightly sealed containers. The powder is stable for 3 years if stored in unopened containers.
Regulatory Status
Included in the FDA Inactive Ingredients Database (topical gel preparation).
Sodium hyaluronate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ZhenYiBio Technology Inc | +8615309206328 | alexxue@zhenyibio.com | China | 298 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21667 | 55 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2966 | 58 |
| Across Biotech Jinan Co LTD | +8613031735486 | frank@acrossbiotech.com | China | 105 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 | kyra@quanjinci.com | China | 1534 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
| Shanghai Likang New Materials Co., Limited | +86-16631818819 +86-17736933208 | 3684455296@qq.com | China | 9311 | 58 |
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 | info@fortunachem.com | China | 5987 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5873 | 58 |
| Kindchem(Nanjing)Co.,Ltd | +86-025-025-85281586 +8618651653755 | sales@kindchem.cn | China | 1227 | 58 |
Related articles
- Sodium hyaluronate:Synthesis, Physical Form, and Toxicity
- Sodium hyaluronate, also known as hyaluronic acid sodium and hyaluronate sodium, is the sodium salt of hyaluronic acid.
- Mar 4,2024
- What is Sodium hyaluronate used for?
- Sodium hyaluronate (NaHA) is a derivative of hyaluronic acid. It has a wide range of uses in medicine, skin care and cosmetics....
- Dec 27,2023
- Sodium Hyaluronate in Skin Care: Benefits, Side Effects and Preparation
- Sodium hyaluronate is an ingredient that's extracted from hyaluronic acid. It’s the sodium salt form of hyaluronic acid.
- Apr 7,2023
View Lastest Price from Sodium hyaluronate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-20 | Sodium hyaluronate
9067-32-7
|
US $6.00 / kg | 1kg | 99% | 2000KG/Month | HebeiShuoshengImportandExportco.,Ltd | |
 |
2024-09-20 | Hyaluronic Acid
9067-32-7
|
US $0.00-0.00 / Kg/Bag | 1Kg/Bag | 99% up | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-09-20 | Sodium Hyaluronate
9067-32-7
|
US $0.00-0.00 / Kg/Bag | 1Kg/Bag | 99% up | 20 tons | Sinoway Industrial co., ltd. |
-

- Sodium hyaluronate
9067-32-7
- US $6.00 / kg
- 99%
- HebeiShuoshengImportandExportco.,Ltd
-

- Hyaluronic Acid
9067-32-7
- US $0.00-0.00 / Kg/Bag
- 99% up
- Sinoway Industrial co., ltd.
-

- Sodium Hyaluronate
9067-32-7
- US $0.00-0.00 / Kg/Bag
- 99% up
- Sinoway Industrial co., ltd.
9067-32-7(Sodium hyaluronate)Related Search:
1of4