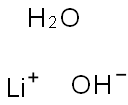Lithium hydroxide monohydrate
- CAS No.
- 1310-66-3
- Chemical Name:
- Lithium hydroxide monohydrate
- Synonyms
- Lithium hydroxide hydrate;ithium hydroxide monohydrate;Lithine hydrate;Lithium hydrate;Monohydrate Lithium Hydroxide;Li.HO.H2O;Lithium hydroxide mo;LITHIUM HYDROXIDE H2O;LITHIUM HYDROXIDE 1HYD;LITHIUM HYDROXIDE H2O ACS
- CBNumber:
- CB6260598
- Molecular Formula:
- Li.HO.H2O
- Molecular Weight:
- 41.96
- MDL Number:
- MFCD00149772
- MOL File:
- 1310-66-3.mol
| Melting point | 462 °C |
|---|---|
| Boiling point | 920 °C |
| Density | 1.51 |
| storage temp. | Store at room temperature. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| color | White to cream or yellow |
| Specific Gravity | 1.51 |
| Odor | Odorless |
| PH Range | 14 |
| PH | ~12 (25℃, 1M in H2O) |
| Water Solubility | 109 g/L (20 ºC) |
| Sensitive | Air Sensitive & Hygroscopic |
| λmax |
λ: 260 nm Amax: 0.02 λ: 280 nm Amax: 0.02 |
| Merck | 14,5534 |
| Stability | Stable. Incompatible with moisture. strong acids, carbon dioxide. Absorbs carbon dioxide from the air. |
| InChIKey | WMFOQBRAJBCJND-UHFFFAOYSA-M |
| CAS DataBase Reference | 1310-66-3(CAS DataBase Reference) |
| FDA 21 CFR | 175.390 |
| EWG's Food Scores | 1-3 |
| FDA UNII | G51XLP968G |
| NIST Chemistry Reference | LiOH(1310-66-3) |
| EPA Substance Registry System | Lithium hydroxide (Li(OH)), monohydrate (1310-66-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H314 | |||||||||
| Precautionary statements | P260-P270-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 22-35-20/22-34-52 | |||||||||
| Safety Statements | 26-36/37/39-45-22-27 | |||||||||
| RIDADR | UN 2680 8/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | OJ6307080 | |||||||||
| F | 3-9-34 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28252000 | |||||||||
| NFPA 704 |
|
Lithium hydroxide monohydrate price More Price(67)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | LX0350 | Lithium Hydroxide, Monohydrate Meets ACS Specifications GR ACS | 1310-66-3 | 500g | $173 | 2024-03-01 | Buy |
| Sigma-Aldrich | LX0350 | Lithium Hydroxide, Monohydrate Meets ACS Specifications GR ACS | 1310-66-3 | 2.5kg | $804 | 2024-03-01 | Buy |
| Sigma-Aldrich | 930903 | Lithium hydroxide monohydrate battery grade, ≥99.9% trace metals basis | 1310-66-3 | 100G | $181 | 2024-03-01 | Buy |
| Sigma-Aldrich | 930903 | Lithium hydroxide monohydrate battery grade, ≥99.9% trace metals basis | 1310-66-3 | 1KG | $1000 | 2024-03-01 | Buy |
| Sigma-Aldrich | 254274 | Lithium hydroxide monohydrate 99.95% trace metals basis | 1310-66-3 | 10g | $57.3 | 2024-03-01 | Buy |
Lithium hydroxide monohydrate Chemical Properties,Uses,Production
Chemical Properties
Both lithium hydroxide and lithium hydroxide monohydrate are colorless crystalline solids at ordinary temperatures. Both are strong bases and should be handled with caution in order to avoid caustic burns. Evaporation of a solution of lithium hydroxide under normal conditions of temperature and pressure results in precipitation of the monohydrate which may be readily dehydrated by heating in a vacuum or under cover of an inert gas.
Lithium hydroxide absorbs water from the air and forms lithium hydroxide monohydrate except under dry atmospheric conditions. Two equilibrium vapor pressures for the conversion of lithium hydroxide monohydrate to anhydrous lithium hydroxide are 4 torr at 25°C and 12 torr at 40°C.

Both anhydrous and lithium hydroxide monohydrate absorb carbon dioxide from the air to form lithium carbonate.
The solubility of lithium hydroxide in water is lower than that of the other alkali metal hydroxides. However, lithium hydroxide is a strong base and reacts completely with both weak and strong acids in aqueous solutions.
Alkali
Lithium hydroxide is a strong base, its chemical properties are more similar to that of the hydroxide at group 2 in periodic table of elements, but quite different with the LDHs in the Group 1.The chemical formula is LiOH, molecular weight is 23.95, melting point of 450 ℃, decomposition temperature of 924 ℃ and relative density is 1.46.Its a white tetragonal crystal and has strong causticity and excitability to the skin.It is slightly soluble in ethanol, soluble in water, but the solubility is less than that of the other alkali metal hydroxides.
The monohydrate can be obtained when the lithium hydroxide react with the water vapor in the air or form the crystals in the aqueous solution. It can react with the acidic gas like sulfur dioxide, hydrogen chloride and hydrogen cyanide etc. It can also react with the strong or weak acids in aqueous solution. Absorbing the carbon dioxide in the air can generate lithium carbonate.
Lithium hydroxide is used as grease additives (thickeners, antioxidants, extreme pressure agents) to improve heat resistance, water resistance, stability and mechanical properties. The grease additives can be used in the bearings of car, plane and crane etc; the raw material of the lithium battery electrolyte.
The calcined solid lithium hydroxide can be used as the carbon dioxide absorbent for the crew members at the spacecraft and submarine.In early American, the areospaceplane of mercury, gemini and apollo project are all using the lithium hydroxide as the absorbers. Its has a reliable performance and can can easily absorb carbon dioxide from the gas contained water vapor. The chemical reaction is: 2LiOH + CO2 → Li2CO3 + H2O, the anhydrous lithium hydroxide of only 1g can absorb carbon dioxide with volume of 450ml. Only 750g of the anhydrous lithium hydroxide can imbibe the carbon dioxide exhaled by one person each day.
Further, lithium hydroxide is also widely used as the raw material to prepare the other subject like lithium compound, lithium salt, lithium soaps, lithium grease, alkyd resins, developer of spectral analysis, the additives of alkaline storage battery, photographic developers and the catalyst etc. It can increase the capacity of the battery by 12%~15% and improve the battery’s life for 2~3 times when it used as the additive for alkaline storage battery Alkaline storage battery as the electrolyte additive.
The information aboved is edited by ChemicalBook Wang Xiaonan.
Development history
The lithium hydroxide was used as the carbon dioxide absorbents in the submarine and the inflatable source of army ballon at 1944.
At 1950, 6Li,the isotope of Li, is used as a raw material for producting the thermonuclear weapons like hydrogen bomb. From that time the United States atomic energy industry began to use a large quantity of lithium hydroxide which lead to amazing development of the lithium industry.
Lithium and its compounds are widely used in the military industry and civilian industry due to its unique chemical and physical properties. Aluminum industry is the largest client of lithium. Adding lithium carbonate to the aluminium electrolysis can reduce the electrolyte’s melting point and increase the current efficiency of aluminum electrolyser resulting in the improment of the aluminum production by about 10% and the power consumption reduced by 8 to 14%; in addition, it can also inhibit the discharge of the harmful fluorine by 22% to 38%. In the United States and Europe, the amount of lithium carbonate consumed by the aluminum industry can take 40% of the total amount of lithium.
Uses
- A principal use of lithium hydroxide monohydrate is as a starting material for numerous other lithium chemicals such as lithium fluoride, lithium chloride, lithium bromide and lithium iodide. Since these materials are prepared in aqueous solutions, there is no particular advantage in using the anhydrous hydroxide for the preparation. Other salts of weak and strong acids may be prepared using lithium hydroxide as a starting material. The preparation of anhydrous lithium hydroxide has already been described.
- A major use of lithium hydroxide monohydrate is in the preparation of lithium salts of fatty acids (lithium soaps) which, with mineral oil and other additives, are used to make lithium-based greases3 6 . Such greases are superior to those based on other metal ions. Lithium greases are good lubricants at high temperatures and are particularly good at low temperatures. They also resist the deteriorating effect of water satisfactorily due to the low water solubility of lithium soaps.
- Both lithium hydroxide and lithium hydroxide monohydrate absorb carbon dioxide readily. Anhydrous lithium hydroxide is used effectively as a carbon dioxide absorbent in life support systems in underwater and aerospace applications.
Chemical Reaction
1. sodium hydroxide generates lithium oxide and water at the condition of 600 ℃ and in the absence of air, the chemical reaction equation:
2LiOH = Li2O + H2O
2. Under the heating conditions, the sodium hydroxide react with the magnesium or calcium can generate lithium and the corresponding lithium oxide.
(1) 2LiOH + Mg = 2Li + MgO + H2O
(2) 2LiOH + Ca = 2Li + CaO + H2O
3. Sodium hydroxide react with the chlorine or iodine can generate lithium halide and lithium chloride and secondary lithium halides.
(1) 2LiOH + Cl2 = LiCl + LiOCl + H2O
(2) 2LiOH + I2 = LiI + LiOI + H2O
4. Lithium hydroxide react with zinc can generate the of zinc acid lithium and hydrogen, the chemical reaction equation:
2LiOH + Zn = LiZnO2 + H2
5. Lithium hydroxide react with acid form the neutralization reaction, and generate the corresponding acid lithium salt (or hydrated lithium salt) and water.
(1) 8H2O LiOH+HBO2+7H2O=LiBO2
(2) 2LiOH+H3PO4 = Li2HPO4+2H2O
(3) 2LiOH+H2SO4 = Li2SO4+2H2O
(4) LiOH (excess) +HClO4 = LiClO4+H2O
6. lithium hydroxide react with a mixture of ammonium chloride, and Li2[HgI4]( Dilithium mercury tetraiodide), or calcium chloride, barium chloride role can generate lithium chloride and other subject.
(1) 4LiOH + 2Li2HgI4 + NH4Cl = LiCl + 7LiI + Ohg2NH2I + 3H2O
(2) 2LiOH + CaCl2 = 2LiCl + Ca(OH)2
(3) 2LiOH + BaCl2 = 2LiCl + Ba(OH)2
Preparation
Lithium hydroxide monohydrate may also be made by the evaporation of a solution prepared by the reaction of calcium hydroxide and lithium carbonate.Anhydrous lithium hydroxide may be prepared by heating the monohydrate in air or in a vacuum.
One laboratory preparation of lithium hydroxide or its monohydrate may be carried out by reacting a solution of barium hydroxide with a solution of lithium sulfate, evaporating the resulting solution of lithium hydroxide, and crystallizing lithium hydroxide monohydrate Lithium hydroxide is readily prepared by drying lithium hydroxide monohydrate.
A simple, convenient laboratory technique for preparation of anhydrous lithium hydroxide from the monohydrate involves vacuum drying in a heated desiccator. To avoid forming a sintered anhydrous material, heat should be applied slowly while maintaining a high vacuum. Contact with air is also avoided by such a procedure, and carbonate formation is minimized.
Production method
Lime sinter process: spodumene concentrate (containing 6% of lithium oxide) mixed with limestone and mill the mixture finely. The lithium aluminate and calcium silicate can be obtained after sintering at 1150~1250 ℃.Crushing the product by the wet grinding, and leaching the lithium hydroxide with lotion. After the settling filtration and the evaporation, concentration, crystallization of the leaching solution. The final product-lithium hydroxide can be get
Li2O•AI2O3•4SiO2+8CaO→Li2O•A12O3+4[2CaO•SiO2]
Li2O•AI2O3+Ca(OH)2→2LiOH+CaO•AI2O3
Uses
Used in catalyst for alkyd resins.
Uses
Lithium hydroxide monohydrate is used for the production of lithium greases, lithium soaps, lithium stearate and lithium salts. It finds application as a carbon dioxide adsorbent in breathing gas purification systems for spacecrafts, submarines and rebreathers; as a storage-battery electrolyte; as a heat transfer medium and as a catalyst for polymerization reaction. It is also used in ceramics and some portland cement formulations.
Preparation
Lithium hydroxide monohydrate may also be made by the evaporation of a solution prepared by the reaction of calcium hydroxide and lithium carbonate.Anhydrous lithium hydroxide may be prepared by heating the monohydrate in air or in a vacuum.
General Description
Small colorless crystals. Denser than water. Contact may cause severe irritation to skin, eyes, and mucous membranes. Toxic by ingestion, inhalation and skin absorption. Used to make electric storage batteries, soaps, and lubricants.
An aqueous solution of a lithium salt below its boiling point has been considered for both a tritium breeding blanket and as a coolant in several fusion reactor designs. In order to breed sufficient tritium to fuel the reactor, a high concentration of lithium is desirable and lithium hydroxide is sufficiently soluble for this purpose. The solution would be contained in pipes and heat exchangers, most probably made from carbon steel.
Air & Water Reactions
Soluble in water; heat of dissolution may generate steam and cause spattering.
Reactivity Profile
LITHIUM HYDROXIDE MONOHYDRATE neutralizes acids exothermically to form salts plus water. Reacts with certain metals (such as aluminum and zinc) to form oxides or hydroxides of the metal and generate gaseous hydrogen. May initiate polymerization reactions in polymerizable organic compounds, especially epoxides. May generate flammable and/or toxic gases with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. May serve as a catalyst. Reacts when heated above about 84°C with aqueous solutions of reducing sugars other than sucrose to evolve toxic levels of carbon monoxide [Bretherick, 5th Ed., 1995].
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Purification Methods
It crystallises from hot water (3mL/g) as the monohydrate. It is dehydrated at 150o in a stream of CO2-free air. It sublimes at 220o with partial decomposition [Cohen Inorg Synth V 3 1957, Bravo Inorg Synth VII 1 1963].
Lithium hydroxide monohydrate Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 | kyra@quanjinci.com | China | 1534 | 58 |
| Tianjin Zhongxin Chemtech Co., Ltd. | +86-022-66880623 +8618622897568 | sales@tjzxchem.com | China | 564 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 | catherine@yjchem.com.cn | China | 983 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| Hebei Mojin Biotechnology Co.,Ltd | +86-15028179902 | angelia@hbmojin.com | China | 1085 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12446 | 58 |
| ChemFine International Co.,Ltd. | +86-510-82753588 +86-13806194144 | info@chemfineinternational.com | China | 300 | 58 |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 | admin@jiuzhou-chem.com;jamie@jiuzhou-chem.com;alice@jiuzhou-chem.com | China | 12272 | 58 |
| Hebei Fengjia New Material Co., Ltd | +86-0311-87836622 +86-17333973358 | sales06@hbduling.cn | China | 8054 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
Related articles
- Crystal structure of lithium hydroxide monohydrate
- Lithium hydroxide monohydrate, LiOH· H20, the "lithium hydrate" of commerce, was described crystallographically by V. von Lang....
- Apr 24,2024
- Property, preparation and application of lithium hydroxide monohydrate
- Lithium hydroxide monohydrate is one of the most important lithium compounds, is widely used in chemical raw materials, chemi....
- Jul 5,2022
View Lastest Price from Lithium hydroxide monohydrate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-23 | Lithium Hydroxide Lioh
1310-66-3
|
US $10.00 / kg | 1kg | 98% | 10 ton | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-09-23 | Lithium hydroxide monohydrate
1310-66-3
|
US $58.00-560.00 / kg | 1kg | 0.99 | 50tons | Wuhan Quanjinci New Material Co.,Ltd. | |
 |
2024-08-24 | Lithium hydroxide monohydrate
1310-66-3
|
US $3.00 / kg | 1kg | 0.99 | 10000 | Hebei Fengjia New Material Co., Ltd |
-

- Lithium Hydroxide Lioh
1310-66-3
- US $10.00 / kg
- 98%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- Lithium hydroxide monohydrate
1310-66-3
- US $58.00-560.00 / kg
- 0.99
- Wuhan Quanjinci New Material Co.,Ltd.
-

- Lithium hydroxide monohydrate
1310-66-3
- US $3.00 / kg
- 0.99
- Hebei Fengjia New Material Co., Ltd