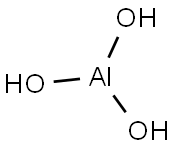
Aluminum hydroxide
- CAS No.
- 21645-51-2
- Chemical Name:
- Aluminum hydroxide
- Synonyms
- ALUMINIUM HYDROXIDE;ALUMINIUM HYDROXIDE GEL;ALUMINUM HYDROXIDE GEL;ALHYDROGEL;c33;ALUMINA HYDRATE;Aluminiumhydroxid;Aluminum hydroxide min;c4d;h46
- CBNumber:
- CB9243680
- Molecular Formula:
- AlH3O3
Lewis structure
3.png)
- Molecular Weight:
- 78
- MDL Number:
- MFCD00003420
- MOL File:
- 21645-51-2.mol
- MSDS File:
- SDS
| Melting point | 300℃ |
|---|---|
| Boiling point | 2980℃[at 101 325 Pa] |
| Density | 2.42 g/cm3 at 20 °C |
| vapor pressure | <0.1 hPa (20 °C) |
| refractive index | Average refractive index: 1.57-1.59 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 0.0015g/l |
| form | colloidal suspension |
| Specific Gravity | 2.42 |
| color | White |
| Odor | Odorless |
| PH Range | >7 |
| PH | 8-9 (100g/l, H2O, 20℃)(slurry) |
| Water Solubility | insoluble |
| Crystal Structure | Monoclinic |
| Merck | 14,342 |
| Solubility Product Constant (Ksp) | pKsp: 32.89 |
| Exposure limits | ACGIH: TWA 1 mg/m3 |
| Dielectric constant | 2.2(Ambient) |
| Stability | Stable. Incompatible with strong bases. |
| LogP | -1.380 (est) |
| Substances Added to Food (formerly EAFUS) | ALUMINUM HYDROXIDE |
| SCOGS (Select Committee on GRAS Substances) | Aluminum hydroxide |
| FDA 21 CFR | 177.1200; 177.2600; 182.90; 310.545 |
| CAS DataBase Reference | 21645-51-2(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 5QB0T2IUN0 |
| NCI Drug Dictionary | aluminum hydroxide |
| ATC code | A02AB01 |
| EPA Substance Registry System | Aluminum hydroxide (21645-51-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | - | |||||||||
| RTECS | BD0940000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28183000 | |||||||||
| Toxicity | TDLo oral in child: 79gm/kg/2Y-I | |||||||||
| NFPA 704 |
|
Aluminum hydroxide price More Price(36)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 239186 | Aluminum hydroxide reagent grade | 21645-51-2 | 2kg | $223 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1017502 | Dried Aluminum Hydroxide Gel United States Pharmacopeia (USP) Reference Standard | 21645-51-2 | 200mg | $460 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.01091 | Aluminium hydroxide powder, hydrargillite EMPLURA? | 21645-51-2 | 1kg | $53.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.01091 | Aluminium hydroxide powder, hydrargillite EMPLURA? | 21645-51-2 | 50kg | $1220 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.01093 | Aluminum hydroxide (fine powder pure, hydrargillite), Technipur? | 21645-51-2 | 1KG | $42.4 | 2023-06-20 | Buy |
Aluminum hydroxide Chemical Properties,Uses,Production
Classifications
Aluminium hydroxides are available in different structures, including aluminium trihydroxide and aluminium oxide hydroxide. Their chemical properties are shown below: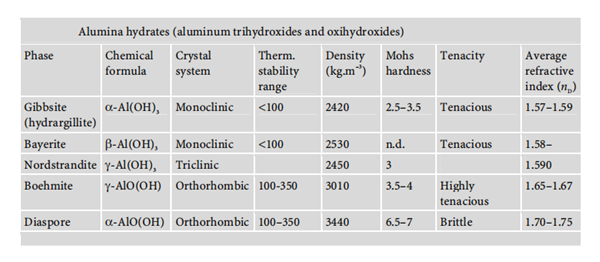
Overview
Aluminum hydroxide, white solid, is a typical amphoteric hydroxide that is insoluble in water but soluble in acid or alkali. It can be transformed into alumina after heated in the air for dehydration, which is important for alumina production. Aluminum hydroxide is a widely used chemical product, and it is mainly used as plastic and polymer fillers, blanket flame retardant and binder, epoxy resin filler, toothpaste fillers, glass ingredients as well as paper color fillers and coatings. It can be also used to product sulfuric acid Aluminum, alum, aluminum fluoride and sodium aluminate, and to synthesize molecular sieve. The gel and drying gel of aluminum hydroxide can be used in medicine as antacids to neutralize gastric acid and protect ulcer surface for the treatment of gastric and duodenal ulcer disease and hyperacidity.
Chemical properties
white crystalline powder; Insoluble in water and alcohol; soluble in inorganic acid and sodium hydroxide solution.
Physical and chemical properties
Chemical formula: Al(OH)3. White crystal. Density: 2.42g•cm-3. Dehydration temperature: 300℃. Insoluble in water. Aluminum hydroxide is a typical amphoteric hydroxide, and it can be soluble in acid to form aluminum salt and soluble in alkali to form aluminate.
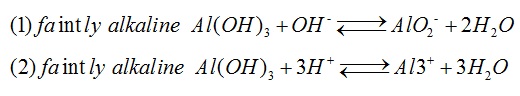
The overview, physical and chemical properties, preparation methods and applications of the aluminum hydroxide are edited by the Eastern editor of Chemicalbook. (2016-12-01)
Preparation
1. Industrial production methods
Industrial production methods include Bayer and sintering methods.
(1) Bayer method
97% of the bauxite ores produced worldwide each year are treated with Bayer method to obtain alumina. The Bayer method consists of two main processes:
1) Treat bauxite with caustic soda solution to transform the alumina in the ore into sodium aluminate.
2) Decompose the cooled sodium aluminate solution by stirring and then separate by filtration to obtain aluminum hydroxide product.

The caustic soda after decomposition can be recycled.
(2) Sintering method
In industrial aluminum, the sintering method is mainly used for high silica bauxite, namely Al2O3/SiO2 <7. The sintering method includes the following three steps:
1) Mix the bauxite and sodium carbonate solution by a certain percentage for sintering.

2) Eluviate the sintered bauxite with sodium carbonate solution to prepare sodium aluminate.

3) Introduce CO2 gas into the sodium aluminate solution to form aluminum hydroxide. At the same time, caustic soda can be transformed into sodium carbonate and then used repeatedly in the sintering process.

2. Hydrothermal method
Aluminum hydroxide synthesized by hydrothermal method has the advantages of high purity, small particle size, uniform distribution, easily-controlling crystal form and simple operation. Therefore, this method is widely used in the process of synthesis of aluminum hydroxide.
3. Sol-gel method
Sol-gel method is commonly used for the preparation of ultra-fine aluminum hydroxide. The most common sol-gel method for the preparation of aluminum hydroxide is the hydrolysis of aluminum salts and alkoxides in water, the mechanism of which is divided into two steps: 1)-OR group is hydrolyzed to produce-OH; 2) Al3+ reacts with –OH to separate aluminum hydroxide precipitation out.
4. Carbon fractionation
Carbon fractionation is a method that is performed as follows: Introduce CO2 gas into sodium metaaluminate solution to make aluminum hydroxide precipitate down and control the size and morphology of products by adjusting the pH value and CO2 concentration.
5. Microemulsion method
Microemulsion is typically composed of surfactants, cosurfactants, solvents and water (or aqueous solution). Microemulsion has many excellent properties such as ultra-low interfacial tension and high solubilization capacity. The preparation of nano-materials by microemulsion technology can precisely control the crystal growth process of nano-materials, and the micro-emulsion ball can encapsulate the crystal particles to effectively prevent the agglomeration of nano-particles.
Uses
1. Chemical raw materials
Aluminum hydroxide has many advantages including large-scale production, adequate raw materials, high product purity and good solubility in acid. Therefore, aluminum hydroxide can be used as an important raw material for the preparation of aluminum salts, such as barium aluminate, aluminum sulfate and so on.
2. Flame retardants
Aluminum hydroxide powder is commonly regarded as an ideal flame retardant filler for plastics, unsaturated polyester, rubber and other organic polymers because of its filling, flame retardant and smoke-eliminating functions and non-toxic property. Flame retardant mechanism of aluminum hydroxide is as follows: when the temperature exceeds 200 ℃, the aluminum hydroxide begin to perform endothermic decomposition and release three crystal water, and its decomposition rate reaches the largest at 250℃.

This reaction is a strong endothermic reaction, thereby inhibiting the polymer temperature rise, reducing its decomposition rate and only producing water vapor, not generating toxic and harmful gases.
3. Ceramics
Aluminum hydroxide can transform into alumina, which has high thermal chemical stability, thermal strength, creep resistance and dielectric properties and low thermal expansion coefficient. Alumina is an important material for the synthesis of ceramics. In the process of ceramic synthesis, we can control the phase formation of the composite by aluminum hydroxide activation and crystallization process controlling.
4. Sewage treatment
Aluminum hydroxide exists in water mainly in form of Al(OH)4-, which can precipitate toxic heavy metals in sewage by coprecipitation method to achieve the effect of water purification after further filter. Aluminum hydroxide has a high specific surface area, and can adsorb colloid, suspended solids, dyes and organic substances in sewage on its surface.
5. Medicine
Aluminum hydroxide can neutralize gastric acid and is non-toxic, for which it is always used as the traditional medicine for the treatment of stomach. The aluminum hydroxide as adjuvant can also improve the immunogenicity of the vaccine, the action mechanism of which is as follows: aluminum hydroxide adsorbs antigen on its surface to allow the antigen slow release so that it can play the role of extending efficacy.
6. Catalyst carrier
In the preparation of aluminum hydroxide, we can obtain various target products with diffrernt surface area, pore volume, pore structure and crystal structure by controlling the temperature, concentration and pH of the reactants, which can be effectively used as a catalyst carrier for the hydrogenation of unsaturated carbonyl compounds and the preparation of fullerenes and the like.
7. Paper industry
Aluminum hydroxide has high whiteness, ultrafine particle size as well as complete crystal form, and has a strong compatibility with brightening agent. Aluminum hydroxide, as an additive coating and resin, can effectively improve the whiteness, opacity, smoothness and ink absorption of coated paper.
Main application
(1) Used for preparing waterproof fabrics, inks, glass, paper fillers, mordant, purifying agent, various aluminum salts, etc.
(2) Widely used for plastics, rubber, resin, paint, paint and so on
(3) Used for supporting catalyst and separating the vapor liquid
(4) Used in the industries of petroleum, chemical, fertilizer, natural gas and environmental protection for increasing the gas or liquid distribution points and protecting the low-strength catalyst.
(5) Used as mordants and analysis reagents.
(6) Used as the thickener for ink and the raw materials for manufacturing aluminum salt, enamel, ceramics, glassware and lubricant. Also used for the preparation of various catalyst carrier. Aluminum hydroxide gel can be used for the treatment of duodenal ulcer, gastric ulcer and hyperacidity embolism. In addition, also used in waterproof fabrics, paper fillers, mordant and purifying agent.
(7) Used for printing inks, painting pigments, crayons and rubber packing.
(8) Uses in waterproof fabric, ink, glassware, paper packing, mordant, purifying agent and also used in aluminum salt, lubricant manufacture.
(9) Used for the gravimetric determination of potassium content. Used as adsorbents, emulsifiers, ion exchangers, chromatographic analytes and mordants. Used for the preparation of refractory, glass and pottery, as well as precipitation pigment and waterproof fabric. Also used for the manufacture of aluminum salts.
Production method
(1) Add alkaline solution to the aluminum sulfate solution under stirring to form precipitation. The precipitation is washed, filtered and dried at low temperature, and then crushed to obtain the finished product. The dewatered paste may also be used as the product directly. The solution concentration, reaction temperature and drying temperature in the preparation process all affect the product quality.
(2) Ammonium bicarbonate method: sulfuric acid reacts with aluminum powder or aluminum ash to generate aluminum sulfate, and then aluminum sulfate perform metathesis reaction with ammonium bicarbonate to obtain aluminum hydroxide.
2Al(OH)3+3H2SO4→A12(SO4)3+6H2O
A12(SO4)3+6NH4HCO3→2AI(OH)3++3(NH4)2SO4+6CO2↑
Sodium aluminate method: caustic soda and aluminum ash react at the ratio of 2: 1 at 100 ° C to obtain sodium aluminate solution. Sulfuric acid and aluminum ash react at the ratio of 1.25: 1 at 110 ° C to produce aluminum sulfate solution. Then, the sodium aluminate solution and the aluminum sulfate solution are neutralized to pH 6.5 to produce aluminum hydroxide precipitate. The obtained precipitate is washed with water, filtered and dried at 70-80℃ for 12 hours, and then crushed to prepare the aluminum hydroxide product.
A12O3+2NaOH→2NaAO2+H2O
Al2O3+3H2SO4→A12(SO4)3+3H2O
6NaAIO2+A12(SO4)3+12H2O→8Al(OH)3↓+3Na2SO4
Recovery method: the recycled aluminum chloride is dissolved in water, decolorized with activated carbon and filtered to remove impurities, and then react with sodium carbonate to produce raw aluminum hydroxide. The raw products are filtered, washed and dried to obtain the final aluminum hydroxide products.
2A1C13+3Na2CO3+3H2O→2AI(OH)3↓+6NaCl+3CO2↑
Solubility in water
Solubility in 100 ml of water: 0.0001 g/20℃
Toxicity
The toxicity of aluminum mainly includes two aspects: the first is the mechanical stimulation to lung tissue; the second is to make protein precipitation and form fiber-like irreversible protein compounds with non-inflammatory performance. The inhalation of aluminum dust can damage the lungs to cause bauxite lung, the chronic symptoms of which have weight loss, easy fatigue, difficult breathing and cough. Aluminum hydroxide is more likely to cause alveolar epithelial hyperplasia than aluminum.The maximum allowable concentration of aluminum hydroxide is 6 mg/m3.
Small trauma can be first treated with alcohol and gasoline, and then covered with non-toxic dressing; large wound can be excised and sutured, and then treated with sulfonamides and penicillin therapy. People working in dusty places should wear gas masks, protective glasses and dust protective overalls to protect the skin and eyes. In addition, every year people should attend a regular physical examination.
Hazards & Safety Information
Category Toxic substances
Toxicity classification High toxic
Acute toxicity celiac-rat LD50: 150 mg/kg
Storage and transportation characteristics Ventilation; Low temperature; dry
Fire extinguishing agent dry powder, foam, sand, carbon dioxide; fog water
Occupational Standard TWA 2mg (Al)/m3
Description
Aluminum hydroxide, insoluble in water, soluble in dilute mineral acids and alkali hydroxides (4 % suspension in water), white, amorphous powder. For other relevant properties and production, see→Aluminum Oxide, Chap. 1.1. Available as Al(OH)3·nH2O (algedrate) and algedrate hexitol complex.
Chemical Properties
Aluminum hydroxide, Al(OH)3, also known as aluminum trihydroxide, aluminum trihydrate, aluminum hydrate, hydrated alumina, and hydrated aluminum oxide, is a white to whitish-yellow water-insoluble powder with a specific gravity of 2.42. Aluminum hydroxide is used as a baseforpigments, as a water repellent in textile coatings, and as an antacid in medicine. Aluminum hydroxide is soluble in hydrochloric or sulfuric acids or in sodiumhydroxide.
Chemical Properties
Aluminum hydroxide adjuvant is a white hydrogel that sediments slowly and forms a clear supernatant.
Uses
As absorbent, in chromatography, manufacturing of glass, paper, inks, ceramics, lubricants, cosmeticsAluminum hydroxide is used as a desiccant powder, filler in paper, plastics, rubber and cosmetics. It is used as a smoke suppressant and mordant dye. It is also used in drugs as antacid and antihyperphosphatemic. It is also used as a Claus catalyst support for waterproof fabrics. It is an important starting material for the preparation of other aluminum compounds, calcined alumina, aluminum sulfate, polyaluminum chloride, zeolites, sodium aluminate, activated alumina and aluminum nitrate. In addition, it is used as a fire retardant.
Uses
Mainly used as an Active medicament in an Antacid Formulations, also used in manufacturing of Lake Colors, Inks, glass, effluent treatment, and fire retardants.
Uses
aluminum hydroxide is an inorganic compound used to make a product less transparent. It is also used by formulators as a humectant, and to soften, smooth, and protect the skin. In addition it helps control product viscosity. often found in facial masks and make-up preparations.
Production Methods
Aluminum hydroxide adjuvant is prepared by the precipitation of a soluble aluminum salt by an alkali hydroxide, or the precipitation of an alkali aluminate by acid.
Definition
aluminium hydroxide: A white crystalline compound, Al(OH)3; r.d. 2.42-2.52. The compound occurs naturally as the mineral gibbsite (monoclinic). In the laboratory it can be prepared by precipitation from solutions of aluminium salts. Such solutions contain the hexaquoaluminium( III) ion with six water molecules coordinated, [Al(H2O)6]3+. In neutral solution this ionizes:
[Al(H2O)6]3+→H+ + [Al(H2O)5OH]2+
The presence of a weak base such as S2- or CO32- (by bubbling hydrogen sulphide or carbon dioxide through the solution) causes further ionization with precipitation of aluminium hydroxide
[Al(H2O)6]3+(aq) → Al(H2O)3(OH)3(s) + 3H+(aq)
The substance contains coordinated water molecules and is more correctly termed hydrated aluminium hydroxide. In addition, the precipitate has water molecules trapped in it and has a characteristic gelatinous form. The substance is amphoteric. In strong bases the aluminate ion is produced by loss of a further proton:
Al(H2O)3(OH)3(s) + OH-(aq)→
[Al(H2O)2(OH)4]-(aq) + H2O(l)
On heating, the hydroxide transforms to a mixed oxide hydroxide, AlO.OH (rhombic; r.d. 3.01). This substance occurs naturally as diaspore and boehmite. Above 450℃ it transforms to γ-alumina.
In practice various substances can be produced that are mixed crystalline forms of Al(OH)3, AlO.OH, and aluminium oxide (Al2O3) with water molecules. These are known as hydrated alumina. Heating the hydrated hydroxide causes loss of water, and produces various activated aluminas, which differ in porosity, number of remaining -OH groups, and particle size. These are used as catalysts (particularly for organic dehydration reactions), as catalyst supports, and in chromatography. Gelatinous freshly precipitated aluminium hydroxide was formerly widely used as a mordant for dyeing and calico printing because of its ability to form insoluble coloured lakes with vegetable dyes.
brand name
Amphojel (Wyeth-Ayerst); Dialume (Rhone-Poulenc Rorer).
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Aluminum hydroxide adjuvant is used in parenteral human and veterinary vaccines.It activates Th2 immune responses, including IgG and IgE antibody responses. It is also used for the isolation of certain serum components such as blood clotting factors.
Pharmaceutical Applications
Aluminium hydroxide (Al(OH)3) has several medical applications. It is used as an antacid for treating
heartburn as well as acid indigestion (reflux oesophagitis). It is also known to have healing properties of peptic ulcers. In patients suffering from kidney failure, who show elevated serum phosphate levels (hyperphosphataemia),
Al(OH)3 is used as a phosphate binder.
Al(OH)3 is an amphoteric compound , which means it can react as a base or as an
acid. In its application as an anti-acid, Al(OH)3 reacts with any excess stomach acid (mainly HCl) with the
formation of AlCl3 and water .
Al(OH)3 + 3HCl → AlCl3 + 3H2O
Al(OH)3 is known to cause constipation, so formulations of anti-acids often include a combination with
Mg2+ antacids. Usually, oral antifoaming agents, such as simethicone, are added in order to reduce bloating and discomfort/pain.
Clinical Use
Phosphate binding agent
Antacid
Safety Profile
Poison by intraperitoneal route. Human systemic effects by ingestion: fever, osteomalacia, and gastrointestinal effects. When coprecipitated with bismuth hydroxide and reduced by H2, it is violently flammable in air. Incompatible with chlorinated rubber.
Safety
Aluminum hydroxide adjuvant is intended for use in parenteral vaccines and is generally regarded as nontoxic. It may cause mild irritation, dryness, and dermatitis on skin contact. On eye contact, aluminum hydroxide adjuvant may also cause redness, conjunctivitis, and short-term mild irritation. Ingestion of large amounts may cause gastrointestinal irritation with nausea, vomiting, and constipation. Inhalation of the dried product may cause respiratory irritation and cough. Type I hypersensitivity reactions following parenteral administration have been reported.
Veterinary Drugs and Treatments
Orally administered aluminum hydroxide is used to reduce hyperphosphatemia in patients with renal failure.
Drug interactions
Potentially hazardous interactions with other drugs
Cytotoxics: concentration of dasatinib and erlotinib
possibly reduced - give at least 4 hours before or 2
hours after erlotinib.
Metabolism
Aluminum hydroxide or oxide is slowly solubilised in the stomach and reacts with hydrochloric acid to form aluminium chloride and water. In addition to forming aluminium chloride, dihydroxyaluminium sodium carbonate and aluminium carbonate form carbon dioxide, and aluminium phosphate forms phosphoric acid. About 17-30% of the aluminium chloride formed is absorbed and is rapidly excreted by the kidneys in patients with normal renal function. Aluminium-containing antacids also combine with dietary phosphate in the intestine forming insoluble, nonabsorbable aluminium phosphate which is excreted in the faeces.
storage
Aluminum hydroxide adjuvant is stable for at least 2 years when stored at 4–308℃ in well-sealed inert containers. It must not be allowed to freeze as the hydrated colloid structure will be irreversibly damaged.
Incompatibilities
When exposed to phosphate, carbonate, sulfate, or borate anions, the point of zero charge for aluminum hydroxide adjuvant decreases.
Regulatory Status
GRAS listed. Accepted for use in human and veterinary parenteral vaccines in Europe and the USA. The limits for use in human vaccines are 0.85 mg aluminum/dose (FDA) and 1.25 mg aluminum/ dose (WHO). There are no established limits for use in veterinary vaccines. Reported in the EPA TSCA Inventory.
Aluminum hydroxide Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7845 | 58 |
| Shandong Juchuang Chemical Co., LTD | +86-18885615001 +86-18885615001 | admin@juchuangchem.com | China | 387 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| Yancheng Green Chemicals Co.,Ltd | +undefined-86-25-86655873 | info@royal-chem.com | China | 114 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
Related articles
- Health hazards and Side effects of Aluminum hydroxide
- Aluminum hydroxide treats heartburn, upset stomach, sour stomach, or acid indigestion.
- Apr 15,2024
- What is Aluminium hydroxide used for?
- Aluminium hydroxide, Al(OH)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rare....
- Sep 13,2021
View Lastest Price from Aluminum hydroxide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | Aluminium hydroxide
21645-51-2
|
US $0.00 / KG | 1KG | ≥99.5% | 500mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2024-04-26 | ALUMINIUM HYDROXIDE
21645-51-2
|
US $600.00 / mt | 10mt | 99% | 1000MT | Hebei Yime New Material Technology Co., Ltd. | |
 |
2024-04-26 | Aluminum hydroxide
21645-51-2
|
US $6.00 / kg | 10kg | 76.5% min | 300tons | Hebei Dangtong Import and export Co LTD |
-

- Aluminium hydroxide
21645-51-2
- US $0.00 / KG
- ≥99.5%
- Jinan Finer Chemical Co., Ltd
-

- ALUMINIUM HYDROXIDE
21645-51-2
- US $600.00 / mt
- 99%
- Hebei Yime New Material Technology Co., Ltd.
-

- Aluminum hydroxide
21645-51-2
- US $6.00 / kg
- 76.5% min
- Hebei Dangtong Import and export Co LTD