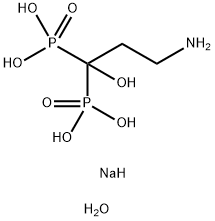
Disodium pamidronate
- CAS No.
- 109552-15-0
- Chemical Name:
- Disodium pamidronate
- Synonyms
- AREDIA;AMINOMUX;GCP-23339A;Aredia (TN);pamidronate,PAD;PAMIDRONATE SODIUM;Disodium Pamidrote;PAMIDRONATE DISODIUM;Disodium pamidronate;Pamedronate disodium salt
- CBNumber:
- CB7707410
- Molecular Formula:
- C3H14NNaO8P2
- Molecular Weight:
- 277.08
- MDL Number:
- MFCD01938386
- MOL File:
- 109552-15-0.mol
| storage temp. | 2-8°C |
|---|---|
| solubility | Soluble in water, practically insoluble in methylene chloride. It is sparingly soluble in dilute mineral acids and dissolves in dilute alkaline solutions. |
| form | White solid |
| CAS DataBase Reference | 109552-15-0(CAS DataBase Reference) |
| FDA UNII | 8742T8ZQZA |
| NCI Dictionary of Cancer Terms | Aredia; pamidronate disodium |
| NCI Drug Dictionary | Aredia |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H319 |
| Precautionary statements | P264-P270-P280-P301+P312-P305+P351+P338-P337+P313 |
| WGK Germany | 3 |
| RTECS | SZ6522666 |
| HS Code | 2931909042 |
| Toxicity | women,TDLo,intravenous,1200ug/kg (1.2mg/kg),BEHAVIORAL: ATAXIAMUSCULOSKELETAL: OTHER CHANGES,Australian and New Zealand Journal of Medicine. Vol. 27, Pg. 719, 1997. |
Disodium pamidronate price More Price(16)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 506600 | Pamidronate, Disodium Salt | 109552-15-0 | 10mg | $142 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1492801 | Pamidronate disodium | 109552-15-0 | 100mg | $260.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | Y0000524 | Pamidronate disodium pentahydrate European Pharmacopoeia (EP) Reference Standard | 109552-15-0 | y0000524 | $220 | 2024-03-01 | Buy |
| American Custom Chemicals Corporation | API0007878 | PAMIDRONATE DISODIUM PENTAHYDRATE 95.00% | 109552-15-0 | 50MG | $700 | 2021-12-16 | Buy |
| Biorbyt Ltd | orb134564 | Pamidronate Disodium >99% | 109552-15-0 | 250mg | $765 | 2021-12-16 | Buy |
Disodium pamidronate Chemical Properties,Uses,Production
Bone absorption inhibitors
Bone metastases are the most common clinical complication in malignant tumors. According to statistics, about 20% -70% of patients with malignant tumors will have bone metastases. The occurrence of bone metastases suggests that tumor progression has reached the advanced stage and the pathological changes caused by bone metastases complications such as fractures have caused great pain to patients. The main clinical symptoms include pain in the transfer site, symptoms of nerve compression, pathological fractures, nerve root damage and spinal cord compression. Therefore, it is very important to relieve the bone pain of patients with bone metastasis and to suppress the occurrence and development of metastasis. Bisphosphonates are the internationally recognized drugs for the treatment of bone metastases. Studies have shown that they can inhibit bone re-sorption, induce osteoclast death, and enhance the activity of osteoclasts caused by the release of various stimulating factors, and relieve the pain of patients with bone pain symptom. Bone metastases is a malignant tumor of other organs transferred to the bone of the tumor, is one of the common clinical tumor complications.
Pamidronate disodium is the second generation of bisphosphonates, studies have shown that pamidronate disodium for cancer-related hypercalcemia and malignant tumors caused by bone pain have a good effect in the treatment of diseases associated with abnormally increased osteoclast activity, and osteolysis caused by advanced tumor bone metastases.
Pamidronate disodium is a bone re-sorption inhibitor with an intensity of 100 times that of etidronate. It can be used for osteoporosis and osteitis deformans caused by various reasons, can eliminate pain, improve exercise capacity and reduce pathological bone. It can also be used for the treatment of malignant tumor caused by hypercalcemia. It has high affinity to the intraosseous hydroxyapatite, can inhibit the dissolution of hydroxyapatite and osteoclast activity. This product can enter into and bind to the hydroxyapatite crystal; when osteoclasts dissolve crystals, the drug will be released to form a protective film to inhibit the activity of osteoclasts; it can also by indirectly inhibit bone re-sorption through osteoblast.
Pharmacological effects
Pamidronate disodium is a potent osteoclastic bone resorption inhibitor. In vitro, it binds tightly to hydroxyapatite crystals and inhibits the formation and dissolution of these crystals. In the body, it can bind to the bone mineral with certain inhibitory effect on the osteoclastic bone resorption. Pamidronate disodium inhibits osteoclast precursor attachment to bone and inhibits its conversion to mature, functional osteoclasts. Local and direct bone resorption effects of bone-bound bisphosphonates are the major mode of action both in vivo and in vitro. Experimental studies have shown that pamidronate disodium can inhibit tumor-induced osteolysis either before or simultaneously with tumor cells inoculated or transplanted. Its inhibitory effect on tumor-induced hypercalcaemia manifested by the following biochemical changes: decreased serum calcium, and secondary reduction of excretion of calcium, phosphate and hydroxyproline in the urine.
Pharmacokinetics: This product is poorly absorbed in the gastrointestinal tract after oral administration; the bioavailability is very low (1% to 3%). After intravenous infusion of this product, steady-state blood concentration is reached within 2 to 3 hours. It is mainly distributed in the bones, liver, spleen and tracheal cartilage with the binding rate with bone being 50% and half-life in bone being 300 days. This product's plasma protein binding rate is 54%, being rapidly eliminated in plasma with plasma half-life of 0.8 to 2 hours. This product is not metabolized in the body, 20% to 55% prototype drugs is excreted from the urine at 72 hours after administration.
Synthetic route
At present, the preparation route of pamidronate disodium is mainly as follows: starting from β-alanine, firstly prepare pamidronate and then prepare pentasodium salt of disodium salt by the following process: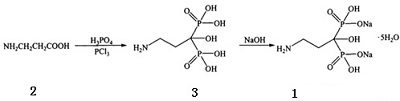
Figure 1 shows the synthetic route of pamidronate
Medicine interactions
- When used in combination with calcitonin, it can produce synergies, leading to a more rapid decrease in serum calcium.
- This product may not be combined with other types of bisphosphonates.
- Owing to the formation of complexes with divalent cations, this product may not be added to calcium-containing intravenous drugs.
Application
For the treatment of bone pain caused by hypercalcemia complicated by malignant tumor and osteolytic cancer metastasis; Degenerative osteitis (Paget's disease) and a variety of osteoporosis; Hyperparathyroidism; tumor bone metastasis-caused excessive dissolution of bone destruction and its complications (such as bone pain pathological fractures, etc.) treatment; also be used for multiple myeloma. For the treatment of hypercalcaemia and osteoporosis.
Adverse reactions
The most common is the short, self-limiting fever; a small number of patients may have increased bone pain at the beginning of medication; also have body malaise, leukopenia. After intravenous administration, patients may have local reactions, thrombophlebitis, chills and so on. Less common adverse reactions include eye reactions such as uveitis, scleritis and conjunctivitis; a small number of patients have allergic reactions such as rashes; gastrointestinal reactions can cause nausea, vomiting, abdominal pain, diarrhea, loss of appetite and constipation.
Precautions
- Patients may have nausea, vomiting, abdominal pain, constipation or diarrhea. Symptoms include chills, chest pain, chest tightness, dizziness and fatigue. Patients of symptomatic esophageal reflux disease, hiatal hernia prone to get esophageal mucosal irritation.
- Intravenous injection or injection can cause transient changes in taste or loss. At 1 to 2 days after injection, it can occur short-term fever.
- High-dose (daily 10 ~ 20mg / kg) can cause bone mineralization disorders, leading to osteomalacia or fracture.
- The drug and bisphosphonates should not be used for allergic history patients. Renal damage patients use with caution. The drug may affect bone growth, and should generally not be used for children.
- During medication process, people should pay attention to monitoring serum calcium, phosphorus, magnesium, potassium and creatinine and so on.
- The injection need administrated through slowly intravenous infusion after calcium-free liquid dilution in order to reduce nephrotoxicity; intravenous injection time should be maintained at least 2 hours, can’t be directly injected intravenously.
Chemical Properties
White or almost white, crystalline powder.
Originator
Aredia,Novartis,India
Uses
Pamidronate Disodium Salt Pentahydrate is a calcium metabolism regulator.
Uses
Bone resorption inhibitor.
Definition
ChEBI: Pamidronate disodium is a 1,1-bis(phosphonic acid).
Manufacturing Process
For a batch size of 5 L, 587.5 g (3.2 moles) of mannitol is dissolved in 3.5 L of water. Pamidronic acid (31.6 g, 0.133 moles) is mixed with a 1.0 L aliquot of the mannitol solution to form a slurry. The slurry is then transferred into the remainder of the mannitol solution, and stirred for at least 15 min. Aqueous 1 N sodium hydroxide (270 ml) is then added and the mixture is stirred until a clear, colorless solution results. The pH is then adjusted to 6.50.1 using either 1 M aqueous phosphoric acid or 1 N aqueous sodium hydroxide, as needed. The solution is then filtered through a 0.22 micron filter, and filled at 20°C into vials at 4.0 ml (4.172 g)/vial, under sterile conditions. The aqueous solution is frozen at -37°C and lyophilized (20 mbar, 20°-40°C) to yield 1,250 vials, each containing 30 mg of amorphous disodium pamidronate. The vials are sealed under positive nitrogen pressure. The disodium pamidronate is amorphous (noncrystalline) by X-ray diffraction and contains 0.7 wt-% water.
brand name
Aredia (Novartis).
Therapeutic Function
Bone resorption suppressant
Clinical Use
Pamidronate, a second-generation bisphosphonate, is 100-fold more potent than etidronate. It has been approved for the treatment of hypercalcemia of malignancy, for Paget's disease, and for osteolytic bone metastases of breast cancer and osteolytic lesions of multiple myeloma. When used to treat bone metastases, pamidronate decreases osteoclast recruitment, decreases osteoclast activity and increases osteoclast apoptosis. Erosive esophagitis has been reported with the use of pamidronate sodium.
Metabolism
Pamidronate is not metabolised, and about 20-55% of the dose is excreted in the urine unchanged within 72 hours; the remainder is mainly sequestered to bone and only very slowly eliminated.
Disodium pamidronate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Shaanxi Yikanglong Biotechnology Co., Ltd. | 17791478691 | yklbiotech@163.com | CHINA | 296 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shanghai Yingrui Biopharma Co.,Ltd | 21-33585366 | export01@shyrchem.com | CHINA | 1320 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
View Lastest Price from Disodium pamidronate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2022-05-14 | Disodium pamidronate
109552-15-0
|
US $1.10 / g | 1g | 99.9% Min | 100 Tons | Dideu Industries Group Limited | |
 |
2021-07-13 | Disodium pamidronate
109552-15-0
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2021-07-09 | Disodium pamidronate
109552-15-0
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- Disodium pamidronate
109552-15-0
- US $1.10 / g
- 99.9% Min
- Dideu Industries Group Limited
-

- Disodium pamidronate
109552-15-0
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- Disodium pamidronate
109552-15-0
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
109552-15-0(Disodium pamidronate)Related Search:
1of4