Aluminum sulfate
- CAS No.
- 10043-01-3
- Chemical Name:
- Aluminum sulfate
- Synonyms
- ALUMINIUM SULPHATE;ALUMINIUM SULFATE;ALUM;Aluminum(III) sulfate;ALUMINIUM SULPAHTE;ALUMINIUM SULPHATE 17% MIN;NA-9078;pearlalum;liusuanlv;filteralum
- CBNumber:
- CB8435192
- Molecular Formula:
- Al2O12S3
- Molecular Weight:
- 342.15
- MDL Number:
- MFCD00003423
- MOL File:
- 10043-01-3.mol
- MSDS File:
- SDS
| Melting point | 770 °C (dec.) (lit.) |
|---|---|
| Boiling point | 759.71°C (estimate) |
| Density | 2.71 g/mL at 25 °C (lit.) |
| vapor pressure | 0-0.001Pa at 20-25℃ |
| solubility | Soluble in cold water, freely soluble in hot water, practically insoluble in ethanol (96 per cent). |
| pka | 3.6[at 20 ℃] |
| form | Powder and/or Chunks |
| Specific Gravity | 2.71 |
| color | White |
| Odor | at 100.00?%. odorless |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,366 |
| Exposure limits | NIOSH: TWA 2 mg/m3 |
| Dielectric constant | 2.6(Ambient) |
| LogP | -5.08--0.12 at 20℃ |
| CAS DataBase Reference | 10043-01-3(CAS DataBase Reference) |
| FDA 21 CFR | 182.1125; 582.1125; 310.545 |
| Substances Added to Food (formerly EAFUS) | ALUMINUM SULFATE |
| SCOGS (Select Committee on GRAS Substances) | Aluminum sulfate |
| EWG's Food Scores | 2 |
| FDA UNII | I7T908772F |
| EPA Substance Registry System | Aluminum sulfate (10043-01-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H290-H318 | |||||||||
| Precautionary statements | P234-P280-P305+P351+P338-P390 | |||||||||
| Hazard Codes | Xi,N | |||||||||
| Risk Statements | 37/38-41-51/53-36/37/38 | |||||||||
| Safety Statements | 26-39-61-37/39-29 | |||||||||
| RIDADR | UN 1760/3077 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | BD1700000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28332200 | |||||||||
| Toxicity | guinea pig,LD50,unreported,490mg/kg (490mg/kg),Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 43(4), Pg. 12, 1978. | |||||||||
| NFPA 704 |
|
Aluminum sulfate price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 202614 | Aluminum sulfate 99.99% trace metals basis | 10043-01-3 | 5g | $89.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 202614 | Aluminum sulfate 99.99% trace metals basis | 10043-01-3 | 25g | $307 | 2024-03-01 | Buy |
| Alfa Aesar | 044563 | Aluminum sulfate, anhydrous, 99.99% (metals basis) | 10043-01-3 | 5g | $53.9 | 2024-03-01 | Buy |
| Alfa Aesar | 044563 | Aluminum sulfate, anhydrous, 99.99% (metals basis) | 10043-01-3 | 25g | $165 | 2024-03-01 | Buy |
| Sigma-Aldrich | 202614 | Aluminum sulfate 99.99% trace metals basis | 10043-01-3 | 100g | $926 | 2024-03-01 | Buy |
Aluminum sulfate Chemical Properties,Uses,Production
Common aluminum salt
Aluminum sulfate is a one of common aluminum salt, prone to hydrolyze, aqueous solution is acid. Added to the solution of soluble carbonates or sulfides will lead to complete hydrolysis:
(1)2Al3++3CO2-3+2H2O=2Al(OH)3↓+3CO2↑ (2)2Al3++3S2-+3H2O=2Al(OH)3↓+3H2S↑
Produce a white flocculent precipitate aluminum hydroxide sediment, when heated it expands violently, becomes spongy substance. Calcined to red heat, then it will be decomposed into sulfur trioxide and alumina.
Aluminum sulfate solution and potassium sulfate solution were mixed, to crystallize, to obtain a new kind of salt called potassium aluminum sulfate K2SO4 · Al2 (SO4) 3 · 24H2O, this salt is called complex salt, also known as aluminum potassium alum, commonly known as alum. From the composition point of view, it is formed by the adduct of two simple salt, is not a simple mixture of the two salt, but the compound of the same crystal structure, certain composition. The difference between complex salt and complexe is that in the solid state or solution complex salt presents simple ions, without complex ions.
Aluminum sulfate and other soluble sulfate, can also form another double salt, the formula of which is M2ⅠSO4MⅡSO4 · 6H2O and MⅠ2SO4M2Ⅲ (SO4) 3 · 24H2O. Where MⅠ typically is NH + 4, Na +, K +, Rb +, Cs +, Tl +.MⅡ1 is Fe2 +, Co2 +, Ni2 +, Zn2 +, Cu2 +, Hg 2+; MuⅢ1 is Fe 3+, Cr3 +, Al3 +, etc. This type of complex salt, collectively known as alum, for example: the molar salt (NH4) 2SO4 · FeSO4 · 6H2O, magnesium potassium sulfate K2SO4 · MgSO4 · 6H2O, chromium potassium sulfate K2SO4 · Cr2 (SO4) 3 · 24H2O, sodium alum Na2SO4 · Al2 ( SO4) 3 · 24H2O, alum, ammonium (NH4) 2SO4 · Al (SO4) 3 · 24H2O, etc. These classes of alum, solubility in water is much smaller than their corresponding sulfates composition, and thus the crystallization from solution, it is easy to get a more complete crystal particles, easily purified. This step of preparing alum is often used for purification of aluminum sulfate or preparation of relatively pure aluminum compound. The class of Alum in the industry has a wide range of uses, alum can be used to clean drinking water, also used for tanning leather, printing and dyeing, papermaking and other industries. Aluminum, aluminum oxide or aluminum hydroxide with sulfuric acid can be prepared for aluminum sulfate.

Lumps Aluminum Sulfate
Physical and Chemical Properties
Colorless or white crystals. Odorless, slightly sweet taste. Because of containing iron etc, industrial product looks like a yellowish-green, tastes sour. It was Stable in the air, heated to 250 ℃ to lose crystal water, when heated above 700 ℃, begin decompose into aluminum oxide, sulfur trioxide and water vapor, etc. Soluble in water, aqueous solution is acidic. Al2 (SO4) 3 + 2H2O → Al2 (SO4) 2 (OH) 2 + H2SO4, when hydrate were heated, violently swell and become spongy, when heated to a red heat, it decomposes into sulfur trioxide and alumina. Flocculent or sponge-like Al (OH) 3 has a strong adsorption capacity, good absorption of pigments and fibers, and thus used as a mordant in dyeing industry, also used to purify drinking water. In addition, in the paper industry, it can added to the pulp with rosin simultaneously for fiber bonding.
Aluminum sulfate has Anhydride and a variety of patterns of hydrate existing (16,18,27, etc). Among aluminum sulfate, the more stable is anhydrous aluminum sulfate [1] and 18 hydrated aluminum sulfate [2]. [1] is a colorless orthorhombic crystals, shiny, or white crystalline powder. Relative molecular mass is 342.15. The relative density is 2.71. Gradually heating to melt, it begins to lose crystal water at 250 ℃, decompose at 770 ℃. The refractive index is 1.47. Dissolved in water, slightly soluble in ethanol, aqueous solution is acidic. Solubility in water: 37.9 at 0 ℃, 38.1 at 10 ℃, 38.5 at 25 ℃, 38.9 at 30 ℃, 40.4 at 40 ℃,44.9 at 60 ℃, 48.8 at 70 ℃, 89 at 100 ℃. [2] is a colorless monoclinic needles. Relative molecular mass is 666.43. The relative density is 1.69 (17/4 ℃). decompose at 86.5 ℃. Dissolved in water, not soluble in ethanol. The solubility in water is 86.9 at 0 ℃, 1104 at 100 ℃. Aqueous solution is acidic due to hydrolysis.
Aluminum sulfate easily absorb water to agglomerate, use plastic bags lined with plastic bags of glass for packaging. Storage and transportation should prevent rain and damp.
Purpose and effect of aluminum sulfate
Aluminum sulfate is mainly used for turbidity water purification, also used as a precipitating agent, fixing agent, fillers, etc. It is used as antiperspirant cosmetic ingredients (astringent) in cosmetics. In addition, aluminum sulfate can also be used as paper industry sizing agent (rosin sizing, so that the color will be attached to the paper), leather tanning agents, mordant, purifying agent (flocculant, the resulting aluminum hydroxide floc may be coated with microparticles suspended in the water to promote rapid sedimentation separation), foam fire extinguisher inside agent (outside agent is sodium bicarbonate, carbon dioxide is generated after the reaction), raw materials for manufacturing alum, and white aluminum, oil bleaching, deodorization agent; pharmaceutical raw materials, as food curing agent (excipient); for impurities removal of oil, water, etc, pasteurized albumin stabilizer, lake raw materials, waste water treatment agent, waterproof concrete materials, fireproof raw materials.
Reference quality standards
Aluminum sulfate Quality Standards (HG1-32-77)
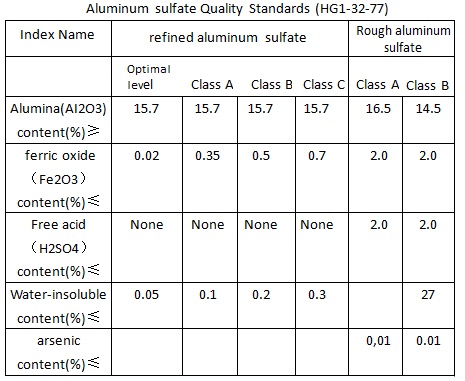
1, All levels of refined aluminum sulfate can be used for producing all levels of precipitation paper sizing agents, class A, B and C is used for water purification, in which arsenic (AS2O3) content should be not more than 0.01%.
2, Aluminum sulfate size: all through the 10 mm mesh sieve, among which the 8 mm sieve fine is not less than 98%, but can also be made bulk product according to user`s requirements.
3, Appearance and color of refined aluminum sulfate is white.
4, All levels of crude aluminum sulfate can be used for water purification, aluminum sulfate used for purification can not be produced by waste acid toxic substances.
5, Aluminum sulfate used in paper sizing, Al2O3 content should be more than 12%, iron content less than 0.2%, insolubles should be not more than 0.5%. Fine paper production requires iron content of aluminum sulfate should be less than 0.08%, this kind of aluminum sulfate is called iron-free aluminum sulfate.
Aluminum sulfate binder
Aluminum sulfate refractory binder is a kind of binding agents. It is industrial aluminum sulfate produced through decomposition of bauxite with sulfuric acid or decomposition of aluminum hydroxide with sulfuric acid, obtained by hydrolysis.
Hardening mechanism of combination of aluminum sulfate binder is more complex, aluminum sulfate solution at room temperature exhibits SO42-, Al (OH) 2+ and Al (SO4) 3 and other states. If left coagulant, coagulated slowly or not solidified. After adding coagulant, such as after adding a coagulant alumina cement, SO42-will seize Ca2 + of alumina cement to form CaSO4, and also form iron sulfate, and magnesium sulfate, etc. The interaction between them would form sulphoaluminate (3CaO · Al2O3 · 3CaSO4 · 31H2O or 3CaO · Al2O3 · CaSO4 · 12H2O) and ferric sulfate (FeO · Al2O3 · 4SO3 · 22H2O) and other precipitation crystallization of new substances, prompting condensation and hardening of combination.
Due to the small number of new product, aluminum sulfate binding agent combination, it is very low intensity at room temperature. Before 500~600 ℃ and after drying heat, treatment intensity strength approaches. When at 700~800 ℃, as aluminate sulfate and sulphoaluminate begin to decompose, releasing SO3, [Al2 (SO4) 3-→ Al2O3 + 3SO3 ↑] to make the body produce binding loose structure, strength decreases. When above 1000 ℃, as the decomposition of aluminate sulfate and sulphoaluminate , the active Al2O3, prone to solid phase reaction, producing new substances and emergence of a liquid phase sintering, the strength improved significantly. when To 1200 ℃, the strength of combination is 3.5 to 5 times the strength of about 800 ℃.
Since aluminum sulfate hydrolysis reaction produces H2SO4, it can react with the raw material metal and oxide to produce hydrogen and water, adversely for brick material molding performance, so when aluminum sulfate binder ingredients are used for burndening, required at a certain temperature and humidity trapped material more than 24h .
Aluminum sulfate binder can be prepared castable, ramming material, plastic and unfired brick, ,etc. Its operating temperature varies as the aggregate and powder materials vary.
Solubility in water (g / 100ml)
Dissolved per 100 ml of water at different temperatures (℃) in grams:
31.2g/0 ℃; 33.5g/10 ℃; 36.4g/20 ℃; 40.4g/30 ℃; 45.8g/40 ℃
59.2g/60 ℃; 73g/80 ℃; 80.8g/90 ℃; 89g/100 ℃
Identification test
With 10% of the sample solution, the aluminum (IT-2) and sulfate (IT-29) test should be positive.
Solubility is measured by the OT-42 method, Easily soluble in water, insoluble in ethanol.
pH is measured with a glass electrode 5% aqueous solution, the value is 2.9 or more.
Content Analysis
The sample of about 4g is accurately weighed, put into a 250ml volumetric flask, dissolved in water and mixed in constant volume. The solution of 10ml is Drew, transferred into a 250ml beaker, adding 0.05mol/L disodium EDTA solution of 25.0ml and pH of 4.5 buffer (made by mixing ammonium acetate of 77.1g and acetic acid of 57ml , adding water to constant volume of 1000m1) 20ml, boiled by slow fire for 5min . After cooling, adding ethanol of 50ml and dithizone test solution (TS-94) of 2ml. Titration With 0.05mol/L zinc sulfate to the bright red rose, while conducting a blank titration. 0.05mol/L disodium EDTA equivalent amounts to aluminum sulfate [Al2 (SO4) 3] of 8.554mg, or aqueous aluminum sulfate [Al2 (S O4) 3 ·18H2O] of 16.66mg.
Toxicity
ADI not specified (FAO/WHO, 2001).
GRAS (FDA, §182.1125,2000).
LD50 6207mg/kg (mice, by mouth)
Uses
Aluminum sulfate is one of the components of Nuclear Fast Red solution.It is a coagulant used for arsenate removal from water.
Aluminum sulfate has been used in the preparation of the nuclear-fast red solution for red nuclear counterstaining in histology and cytology protocols. It may be used as a catalyst for the conversion of lactic acid to acetaldehyde via decarbonylation.
Aluminum sulfate may be used to form a porous composite adsorbent with graphene hydrogel to be used for fluoride removal from water.
The method of producing
After aluminum hydroxide a post (or pure bauxite or kaolin) with sulfuric acid reacted, insolubles were filtered off and then crystallized.
Sulfuric acid production process is by grinding bauxite to a certain particle size, added into reactor for reaction with sulfuric acid, settling the reaction liquid, the supernatant was added sulfuric acid to neutral or slightly alkaline, then concentrated to about 115 ℃, solidified by cooling , crushing prepared products. A12O3 + 3H2SO4 → A12 (SO4) 3 + 3H2O
Category
toxic substances
Toxicity grading
poisoning
Acute oral toxicity
oral-mouse LD50: 6207 mg/kg; intraperitoneal-Mouse LD50: 1735 mg/kg
Flammability hazard characteristics
heat decomposition of toxic sulfur oxides, hydrolysis produces sulfuric acid, lung irritation
Storage characteristics
Treasury ventilation low-temperature drying
extinguishing agents
Foam, carbon dioxide, dry powder
Chemical Properties
Aluminum sulfate is a white powder, often used in water solution. The solution is a strong acid
Physical properties
White powder; refractive index 1.47; density 2.71 g/cm3; mp 770°C (decomposes); hygroscopic; readily soluble in water (31% at 0°C; solubility increases with temperature 98% in boiling water); soluble in dilute mineral acids; slightly soluble in alcohol.
Occurrence
It occurs in nature in minerals; alunite, KAl3(SO4)2(OH)6and natroalunite, NaAl3(SO4)2(OH)6. The anhydrous salt is used in food applications.
Uses
Aluminum sulfate acts as a flocculating agent in the purification of drinking water, and in waste water treatment plants. It acts as a mordant in dyeing, printing, textiles and also used in paper manufacturing. It is a waterproofing agent and accelerator in concrete. Further, it is used as a foaming agent in fire fighting foam, photographic film and in photochemicals. It is also used in styptic pencils, pain relief and in dentistry for gingival retraction cords.
Uses
Sizing paper, lakes, alums, dyeing mordant foaming agent in fire foams, cloth fireproofing, white leather tannage, catalyst in manufacturing ethane, p H control in paper industry, waterproofing agent for concrete, clarifier for fats and oils, lubricating compositions, deodorizer and decolorizer in petroleum refining, sewage precipitating agent and for water purification, food additive.
Definition
alunogenite: A mineral form of hydrated aluminium sulphate, Al2(SO4)3.18H2O.
Preparation
The anhydrous salt may be obtained by slow and progressive heating of commercial hydrated salt, Al2(SO4)3 ?18H2O. Most water molecules are lost at heating between 250 to 420°C. The last three water molecules are lost between 250 to 420°C at a heating rate of 10°C/min.
Definition
ChEBI: An aluminium sulfate that contains no water of crystallisation.
General Description
Anhydrous aluminum sulfate is a white crystalline solid. Aluminum sulfate is also obtained as an 18-hydrate Al2(SO4)3.18H2O. Both forms are soluble in water, noncombustible, and nontoxic. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Aluminium sulfate is used in paper making, in firefighting foams, and in sewage treatment and water purification.
Air & Water Reactions
Dissolves in water with evolution of some heat. creates acidic solutions.
Reactivity Profile
Aqueous solutions of ALUMINUM SULFATE are acidic. The solid may corrode metals in presence of moisture.
Health Hazard
Inhalation of dust irritates nose and mouth. Ingestion of large doses causes gastric irritation, nausea, vomiting, and purging. Dust irritates eyes and skin.
Agricultural Uses
Alunogenite is a naturally occurring form of hydrated aluminum sulphate Al2(SO4)318 H2O.
Industrial uses
Aluminum chloride (AlCl3) can be obtained by reacting carbon dioxide and chlorine with kaolin at high temperatures. It is highly hygroscopic with a specific gravity of 2.3. It is highly soluble in water and in organic solvents. Similar to aluminum sulfate, aluminum chloride is used as a co-depressant for calcite, fluorite and dolomite.
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. Experimental reproductive effects. Human mutation data reported. Hydrolyzes to form sulfuric acid, whch irritates tissue, especially lungs. When heated to decomposition it emits toxic fumes of SOx,.
Potential Exposure
Widely used in the paper industry, in waste and water treatment and treating sewage; in antiperspirants, deodorants; in flame-retardants; in tanning leather, sizing paper; mordant in dyeing, purifying water, waterproofing cloth, clarifying oils and fats; in agricultural pesticides; manufacturing aluminum salts and others
Shipping
UN3264 Corrosive liquid, acidic, inorganic, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required
Purification Methods
It crystallises from hot dilute H2SO4 (l mL/g) on cooling in ice. When a solution of alumina (Al2O3) in conc H2SO4 is slowly cooled, Al2SO4 17 or 18H2O deposits as a crystalline mass. Al2SO4 17H2O is the stable form in equilibrium with its saturated aqueous solution at 25o [Smith J Am Chem Soc 64 41 1942]. This is purified by dissolving it in a small volume of H2O and adding EtOH until the sulfate readily crystallises from the oily supersaturated solution. It forms Al2O3 16H2O between 0-112o. On gradual heating, the hydrate melts, giving the anhydrous salt at ca 250o. Several hydrates up to 27H2O have been described. Further heating to red heat (~ 600-800o) causes decomposition to Al2O3 + SO3 + SO2 and O2 [Cobb J Soc Chem Ind 29 250 1910]. The ACS reagent is Al2O3 18H2O (98+%).
Incompatibilities
In aqueous solution, aluminum sulfate forms sulfuric acid; reacts with bases and many other substances. Corrodes metals, some plastics and body tissues, especially in the presence of moisture.
Waste Disposal
Pretreatment involves hydrolysis followed by neutralization with NaOH. The insoluble aluminum hydroxide formed is removed by filtration and can be heated to decomposition to yield alumina which has valuable industrial applications. The neutral solution of sodium sulfate can be discharged into sewers and waterways as long as its concentration is below the recommended provisional limit of 250 mg/L
Aluminum sulfate Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-13102810335 | admin@hbsaisier.cn | China | 747 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Wuhan Han Sheng New Material Technology Co.,Ltd | +8617798174412 | admin01@hsnm.com.cn | China | 2118 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7845 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Cangzhou Wanyou New Material Technology Co.,Ltd | 18631714998 | sales@czwytech.com | CHINA | 906 | 58 |
Related articles
- Property and Application of Aluminum Sulfate
- Anhydrous aluminum sulfate is a white crystalline solid. Aluminum sulfate is also obtained as an 18-hydrate Al2(SO4)3.18H2O. B....
- Jul 7,2022
View Lastest Price from Aluminum sulfate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | Aluminium sulfate
10043-01-3
|
US $10.00 / kg | 1kg | 99.7% | 200000kg | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2024-04-26 | Aluminum sulfate
10043-01-3
|
US $10.00 / kg | 1kg | 99% | 300tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-26 | Aluminum sulfate
10043-01-3
|
US $170.00 / ton | 5ton | 99% | 3000tons | Hebei Dangtong Import and export Co LTD |
-

- Aluminium sulfate
10043-01-3
- US $10.00 / kg
- 99.7%
- Ouhuang Engineering Materials (Hubei) Co., Ltd
-

- Aluminum sulfate
10043-01-3
- US $10.00 / kg
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Aluminum sulfate
10043-01-3
- US $170.00 / ton
- 99%
- Hebei Dangtong Import and export Co LTD