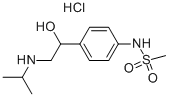
Sotalol hydrochloride
- CAS No.
- 959-24-0
- Chemical Name:
- Sotalol hydrochloride
- Synonyms
- DAROB;SOTALEX;SOTACOR;MJ-1999;BETAPACE;DL-MJ 1999;SOTALOL HCL;SOLATOL HCL;Betapace AF;meadjohnson1999
- CBNumber:
- CB9712098
- Molecular Formula:
- C12H21ClN2O3S
- Molecular Weight:
- 308.82
- MDL Number:
- MFCD00242937
- MOL File:
- 959-24-0.mol
| Melting point | 218-220°C |
|---|---|
| storage temp. | 2-8°C |
| solubility | H2O: 20 mg/mL |
| form | powder |
| color | white to off-white |
| Water Solubility | Soluble in phosphate buffered saline, DMSO, ethanol, water, and methanol. |
| CAS DataBase Reference | 959-24-0(CAS DataBase Reference) |
| FDA UNII | HEC37C70XX |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H303-H315-H319-H335 |
| Precautionary statements | P261-P280a-P304+P340-P405-P501a-P305+P351+P338 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
| RTECS | PB0826000 |
| HS Code | 2935904000 |
| Toxicity | LD50 in male mice, rats (mg/kg): 2600, 3450 orally; 670, 680 i.p.; LD50 orally in rabbits: 1000 mg/kg; LD50 i.p. in dogs: 330 mg/kg (Lish) |
Sotalol hydrochloride price More Price(39)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHR2798 | Sotalol Hydrochloride | 959-24-0 | 500MG | $287 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1617408 | Sotalol hydrochloride United States Pharmacopeia (USP) Reference Standard | 959-24-0 | 300mg | $311.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 39863 | (±)-Sotalol hydrochloride analytical standard | 959-24-0 | 10mg | $144 | 2022-05-15 | Buy |
| Alfa Aesar | J63772 | Sotalol hydrochloride, 98% | 959-24-0 | 1g | $520 | 2024-03-01 | Buy |
| Alfa Aesar | J63772 | Sotalol hydrochloride, 98% | 959-24-0 | 5g | $858 | 2024-03-01 | Buy |
Sotalol hydrochloride Chemical Properties,Uses,Production
Description
Sotalol (hydrochloride) (Item No. 26291) is an analytical reference standard categorized as a β-adrenergic receptor antagonist. It has been detected as an adverse analytical finding (AAF) during anti-doping testing. This product is intended for use in analytical forensic applications. This product is also available as a general research tool .
Description
Sotalol is a non-selective antagonist of β-adrenergic receptors (β-ARs; IC50s = 8.9 and 5.2 μM for β1- and β2-ARs, respectively) and a class III antiarrhythmic agent. It decreases delayed outward potassium currents (IK) in guinea pig ventricular cells and prolongs action potential duration in electrically stimulated isolated guinea pig papillary muscles when used at a concentration of 100 μM. Sotalol decreases heart rate and increases blood pressure and the cardiac functional refractory period (FRP) in a canine model of ventricular tachycardia induced by programmed electrical stimulation (PES). Formulations containing sotalol have been used in the treatment of ventricular arrhythmias and maintenance of normal sinus rhythm in patients with atrial fibrillation or flutter (AFIB/AFL).
Chemical Properties
White Crystalline Solid
Originator
Sotagard,Glaxo
Uses
antibacterial
Uses
Sotalol hydrochloride is used as a potent beta adrenergic antagonist, prolongs the action potential and increases the refractory period. Sotalol hydrochloride is also considered a non-selective β blocker and a potassium channel blocker with an IC50 of 43 μM.
Uses
A potent α-adrenergic receptor antagonist. A class III antiarrythmic. It has been shown to prolong action potential and increases the refractory period.
Uses
A potent -adrenergic receptor antagonist. A class III antiarrythmic. It has been shown to prolong action potential and increases the refractory period
Definition
ChEBI: A hydrochloride salt that is the monohydrochloride of sotalol. It has both beta-adrenoreceptor blocking (Vaughan Williams Class II) and cardiac action potential duration prolongation (Vaughan Williams Class III) antiarrhythmic properties. It is used (usually as the hydrochloride salt) for the management of ventricular and supraventricular arrhythmias.
Manufacturing Process
As a resulting of reaction of methansulfonylchloride reacted with aniline
methansulfonanilide was obtained. The methansulfonanilide reacted with
bromacetylbromide at the presence of AlCl3 and CS2 and 4-(bromacetyl)-
methansulfonanilide was prepeared.
Then to the 4-(bromacetyl)-methansulfonanilide isopropylamine was added to
give 4-(1-oxo-2-isopropylaminoethyl)methansulfonanilide.The 4-(1-oxo-2-isopropylaminoethyl)methansulfonanilide was reduced by
hydrogenesation in the presence of Pd-C catalyst and sodium borohydride. So
4-(1-hydroxy-2-isopropylaminoethyl)methansulfonanilide was obtained.
The 4-(1-hydroxy-2-isopropylaminoethyl)methansulfonanilide hydrochloride
may be prepared by treatment of base with hydrochloric acid.
brand name
Betapace(Berlex); Sorine (Upsher Smith).
Therapeutic Function
Beta-adrenergic blocker, Antiarrhythmic
Biological Activity
A relatively potent pure β adrenergic antagonist, unique in possessing additional class III antiarrhythmic activity. Also available as part of the Mixed Adrenergic Tocriset™ .
Clinical Use
Beta-adrenoceptor blocker:
Treatment of life-threatening ventricular
arrhythmias
Prophylaxis of SVT
Veterinary Drugs and Treatments
Sotalol may be useful in the treatment of ventricular tachycardias and, possibly, supraventricular tachycardias in dogs.
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: NSAIDs antagonise hypotensive effect.
Anti-arrhythmics: increased risk of myocardial
depression and bradycardia; increased risk ofbradycardia, myocardial depression and AV block
with amiodarone; increased risk of ventricular
arrhythmias with amiodarone, dronedarone,
disopyramide or procainamide - avoid; increased
risk of myocardial depression and bradycardia with
flecainide.
Antibacterials: increased risk of ventricular
arrhythmias with moxifloxacin - avoid; increased
risk of ventricular arrhythmias with delamanid.
Antidepressants: enhanced hypotensive effect with
MAOIs; increased risk of ventricular arrhythmias
with tricyclics; increased risk of ventricular
arrhythmias with citalopram, escitalopram and
venlafaxine - avoid.
Antihistamines: increased risk of ventricular
arrhythmias with mizolastine - avoid.
Antihypertensives: enhanced hypotensive effect;
increased risk of withdrawal hypertension with
clonidine; increased risk of first dose hypotensive
effect with post-synaptic alpha-blockers such as
prazosin.
Antimalarials: increased risk of bradycardia with
mefloquine; avoid with artemether and lumefantrine
and piperaquine with artenimol - increased risk of
ventricular arrhythmias.
Antimuscarinics: increased risk of ventricular
arrhythmias with tolterodine.
Antipsychotics: enhanced hypotensive effect with
phenothiazines; increased risk of ventricular
arrhythmias with amisulpride, droperidol,
haloperidol, phenothiazines, pimozide, risperidone,
sulpiride or zuclopenthixol - avoid with droperidol
and zuclopenthixol.
Antivirals: increased risk of ventricular arrhythmias
with saquinavir or telaprevir - avoid.
Atomoxetine: increased risk of ventricular
arrhythmias.
Calcium-channel blockers: increased risk of
bradycardia and AV block with diltiazem;
hypotension and heart failure possible with
nifedipine and nisoldipine; asystole, severe
hypotension and heart failure with verapamil.
Cytotoxics: possible increased risk of bradycardia
with crizotinib; increased risk of ventricular
arrhythmias with vandetanib - avoid; increased risk
of ventricular arrhythmias with arsenic trioxide,
bosutinib, ceritinib, panobinostat and vandetanib.
Diuretics: enhanced hypotensive effect; increased risk
of ventricular arrhythmias due to hypokalaemia.
Fingolimod: possibly increased risk of bradycardia.
Ivabradine: increased risk of ventricular arrhythmias.
Moxisylyte: possible severe postural hypotension.
Ranolazine: avoid concomitant use.
Sympathomimetics: severe hypertension with
adrenaline and noradrenaline and possibly with
dobutamine.
Metabolism
Metabolism of sotalol is negligible, and it is excreted unchanged in the urine.
storage
Store at RT
Sotalol hydrochloride Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 8174 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hebei Jimi Trading Co., Ltd. | +86 319 5273535 | bestoneforyou@sina.com | CHINA | 288 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Standardpharm Co. Ltd. | 86-714-3992388 | overseasales1@yongstandards.com | United States | 14336 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81138252 +86-18789408387 | 1057@dideu.com | China | 3487 | 58 |
View Lastest Price from Sotalol hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-15 | Sotalol hydrochloride
959-24-0
|
US $32.00-1.30 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2022-04-05 | Sotalol Hydrochloride
959-24-0
|
US $1.00-100.00 / gram | 1gram | 99.00%USP | 50kg | Shaanxi Dideu Medichem Co. Ltd | |
 |
2021-08-12 | Sotalol hydrochloride USP/EP/BP
959-24-0
|
US $1.10 / g | 1g | 99.9% | 100 Tons min | Dideu Industries Group Limited |
-

- Sotalol hydrochloride
959-24-0
- US $32.00-1.30 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- Sotalol Hydrochloride
959-24-0
- US $1.00-100.00 / gram
- 99.00%USP
- Shaanxi Dideu Medichem Co. Ltd
-

- Sotalol hydrochloride USP/EP/BP
959-24-0
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited