- Sebacic acid
-

- $100.00 / 50kg
-
2024-09-24
- CAS:111-20-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000Ton
- Sebacic Acid
-

- $100.00 / 1KG
-
2024-09-23
- CAS:111-20-6
- Min. Order: 10KG
- Purity: 99%
- Supply Ability: 10000kg
- Sebacic acid
-

- $9.80/ kg
-
2024-09-13
- CAS:111-20-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10 tons
Related articles - Modern applications of sebacic acid
- Sebacic acid is a naturally occurring dicarboxylic acid that has gained attention for its potential applications in various bi....
- May 31,2023
|
| | Sebacic acid Chemical Properties |
| Melting point | 133-137 °C (lit.) | | Boiling point | 294.5 °C/100 mmHg (lit.) | | density | 1.21 | | vapor pressure | 1 mm Hg ( 183 °C) | | refractive index | 1.422 | | Fp | 220 °C | | storage temp. | Store below +30°C. | | solubility | ethanol: 100 mg/mL | | form | Powder or Granules | | pka | 4.59, 5.59(at 25℃) | | color | White to off-white | | Odor | monoclinic prismatic tablets, wh. powd., fatty acid odor | | Water Solubility | 1 g/L (20 ºC) | | Merck | 14,8415 | | BRN | 1210591 | | Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, bases, reducing agents. | | InChIKey | CXMXRPHRNRROMY-UHFFFAOYSA-N | | LogP | 1.5 at 23℃ | | CAS DataBase Reference | 111-20-6(CAS DataBase Reference) | | NIST Chemistry Reference | Decanedioic acid(111-20-6) | | EPA Substance Registry System | Sebacic acid (111-20-6) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36-24/25 | | WGK Germany | 1 | | RTECS | VS0875000 | | TSCA | Yes | | HS Code | 29171310 | | Toxicity | LD50 orally in Rabbit: 3400 - 14500 mg/kg LD50 dermal Rat > 2000 mg/kg |
| | Sebacic acid Usage And Synthesis |
| The main method of preparation | (1) Castor oil is as raw material, ricinoleate is separated from castor oil, with the condition of inflating and 280~300℃, caustic soda proceeds alkali fusion and the reaction is heated for 10h, sebum acid sodium salt can obtain, deputy product is 2-octanol. The sodium salt is dissolved in water, adding sulfuric acid to neutralize, after bleaching, the solution is cooled to precipitate sebum acid, it is washed with cold water, and finally recrystallized.
CH3 (CH2) 5CH (OH) CH2CH = CH (CH2) 7COOH +
2NaOH → CH3 (CH2) 5CH (OH) CH3 + NaOOC (CH2) 8COONa + H2
NaOOC (CH2) 3COONa + H2SO4 → HOOC (CH2) 8COOH + Na2SO4
(2) Adipic acid (hexane diacid) is as raw material to synthesize. Adipic acid and methanol can proceed esterification reaction to form dimethyl adipate, ion exchange membrane proceeds electrolytic oxidation to get dimer, i.e., dimethyl sebacate, and then reacts with sodium hydroxide to form the disodium salt, hydrochloric acid (or sulfuric acid) is used to neutralize and Sebacic acid can obtain.
Chemical properties, uses and methods of preparation of sebacic acid are edited by Chemicalbook andy.(2016-12-04) | | Chemical properties | It is white flaky crystal. It is slightly soluble in water, soluble in alcohol and ether. | | Uses | Sebacic acid is widely used in the preparation of sebacic acid esters, such as dibutyl sebacate, dioctyl sebacate, diisooctyl sebacate. These esters can be used as plasticizers for plastics and cold-resistant rubber, as well as for polyamide, polyurethane, alkyd resin, synthetic lubricating oil, lubricating oil additives, spices, coatings, cosmetics, etc. It can also be used as raw material for producing nylon 1010, nylon 910, nylon 810, nylon 610, nylon 9 and high temperature resistant lubricating oil diethylhexyl ester. It is also the raw material for the production of alkyd resins (used as surface coatings, plasticized nitrocellulose coatings, and urea resin varnishes) and polyurethane rubber, cellulose resins, vinyl resins, and synthetic rubber plasticizers, softeners, and solvents.
| | Production method | Almost all of the current industrial production of sebacic acid is using castor oil as raw material.
Castor oil cracking method: castor oil is heated under the action of alkali hydrolysis to generate ricinoleic acid sodium soap, and then add sulfuric acid acid to generate ricinoleic acid; in the presence of diluent cresol, add alkali heated to 260-280 ℃ for cracking to generate sebacic acid double sodium salt and secoctanol and hydrogen, cracked material diluted by water, heated and neutralized with acid, the double sodium salt into a monosodium salt; and then boiled with acid after decolorization of activated carbon neutralization solution. The monosodium salt of sebacic acid is turned into sebacic acid crystals, and then separated and dried to obtain the finished product. | | Toxicity | Sebacic acid, also known as 1, 10-decanedioic acid, belongs to aliphatic dibasic acid. Sebacic acid was present in the leaves of flue-cured tobacco, burley tobacco and aromatic tobacco. Sebacic acid was white crystal in flake form at room temperature. Slightly soluble in water, sebacic acid was insoluble in benzene, petroleum ether, carbon tetrachloride. In contrast, sebacic acid was soluble in ethanol and ethyl ether. Irritant to the eyes, respiratory system and skin irritation, sebacic acid oral harmful. However, sebacic acid was low toxic and flammable.
| | Hazards & Safety Information | Category: Flammable liquid
Toxicity: grading toxicity
Acute oral toxicity-rat LD50: 14375 mg/kg; Oral-Mouse LD50: 6000 mg/kg
Flammability hazard characteristics: flammable, the fire discharges acrid smoke
Storage characteristics: Treasury ventilation low-temperature drying
Extinguishing agent: Dry powder, foam, sand, water | | Description | Sebacic acid is a dicarboxylic acid with structure (HOOC)(CH2)8(COOH), and is naturally occurring.
In its pure state it is a white flake or powdered crystal. The product is described as non-hazardous, though in its powdered form it can be prone to flash ignition (a typical risk in handling fine organic powders).
Sebaceus is Latin for tallow candle, sebum (tallow) is Latin for tallow, and refers to its use in the manufacture of candles. Sebacic acid is a derivative of castor oil, with the vast majority of world production occurring in China which annually exports over 20,000 metric tonnes, representing over 90 % of global trade of the product.
In the industrial setting, sebacic acid and its homologues such as azelaic acid can be used in plasticizers, lubricants, hydraulic fluids, cosmetics, candles, etc. Sebacic acid is also used as an intermediate for aromatics, antiseptics, and painting materials. | | Chemical Properties | White flaky crystals. Slightly soluble in water, soluble in alcohol and ether. | | Uses | Sebacic Acid is a urinary metabolite that has been identified as an anti-fatigue biomarker. | | Uses | Decanedioic acid was named by Thenard LJ (1802) from the Latin sebaceus(tallow candle) or sebum (tallow) in reference to its use in the manufacture of candles. Thenard LJ isolated this compound from distillation products of beef tallow. In 1954, it was reported that it was produced in excess of 10,000 tons annually by alkali fission of castor oil. Sebacic acid and its derivatives, as azelaic acid, have a variety of industrial uses as plasticizers, lubricants, diffusion pump oils, cosmetics, candles, etc. It is also used in the synthesis of polyamide, as nylon, and of alkyd resins. An isomer, isosebacic acid, has several applications in the manufacture of vinyl resin plasticizers, extrusion plastics, adhesives, ester lubricants, polyesters, polyurethane resins and synthetic rubber. | | Definition | ChEBI: Sebacic acid is an alpha,omega-dicarboxylic acid that is the 1,8-dicarboxy derivative of octane. It has a role as a human metabolite and a plant metabolite. It is an alpha,omega-dicarboxylic acid and a dicarboxylic fatty acid. It is a conjugate acid of a sebacate(2-) and a sebacate. It derives from a hydride of a decane. | | Preparation | Sebacic acid is normally made from castor oil, which is essentially glycerol
triricinoleate. The castor oil is heated with sodium hydroxide at about 250??e.
This treatment results in saponification of the castor oil to ricinoleic acid
which is then cleaved to give 2-octanol and sebacic acid:
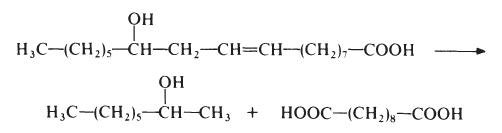
This process results in low yields of sebacic acid (about 50% based on the
castor oil) but, nevertheless, other routes have not proved competitive.
Sebacic acid is a colourless crystalline solid, m.p. 134??. | | General Description | White granular powder. Melting point 153°F. Slightly soluble in water. Sublimes slowly at 750 mm Hg when heated to melting point. | | Air & Water Reactions | Insoluble in water. | | Reactivity Profile | Sebacic acid reacts exothermically to neutralize bases, both organic and inorganic. May react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Can react with active metals to form gaseous hydrogen and a metal salt. Such reactions are slow in the dry, but systems may absorb enough water from the air to allow corrosion of iron, steel, and aluminum parts and containers. Reacts slowly with cyanide salts to generate gaseous hydrogen cyanide. Reacts with solutions of cyanides to cause the release of gaseous hydrogen cyanide. May generate flammable and/or toxic gases and heat with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. May react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Can be oxidized exothermically by strong oxidizing agents and reduced by strong reducing agents. May initiate polymerization reactions. | | Fire Hazard | Flash point data for Sebacic acid are not available. Sebacic acid is probably combustible. | | Flammability and Explosibility | Not classified | | Purification Methods | Purify sebacic acid via the disodium salt which, after crystallisation from boiling water (charcoal), is again converted to the free acid. The free acid is crystallised repeatedly from hot distilled water or from Me2CO/pet ether and dried under vacuum. [Beilstein 2 IV 2078.] |
| | Sebacic acid Preparation Products And Raw materials |
|