- Bromfenac sodium
-

- $0.00 / 1KG
-
2023-11-27
- CAS:91714-93-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20 TONS
- Bromfenac sodium
-

- $15.00 / 1KG
-
2021-07-13
- CAS:91714-93-1
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Bromfenac sodium
-

- $15.00 / 1KG
-
2021-07-09
- CAS:91714-93-1
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
|
| | Bromfenac sodium Basic information |
| Product Name: | Bromfenac sodium | | Synonyms: | 2-AMINO-3-(4-BROMOBENZOYL)PHENYLACETIC ACID SODIUM;2-Amino-3-(4-bromobenzoyl)phenylacetic acid sodium salt;Sodium [2-amino-3-(4-bromobenzoyl)phenyl]acetate;Ahr 10282b;Benzeneacetic acid, 2-amino-3-(4-bromobenzoyl)-, monosodium salt;Bronuck;2-{2-aMino-3-[(4-broMophenyl)carbonyl]phenyl}acetic acid;AHR 10282R | | CAS: | 91714-93-1 | | MF: | C15H11BrNNaO3 | | MW: | 356.15 | | EINECS: | 804-379-0 | | Product Categories: | Inhibitors;Aromatic Phenylacetic Acids and Derivatives | | Mol File: | 91714-93-1.mol | 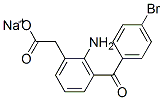 |
| | Bromfenac sodium Chemical Properties |
| Melting point | 285 ºC | | storage temp. | 2-8°C | | solubility | H2O: ≥5mg/mL | | form | powder | | color | faint yellow to dark yellow | | InChIKey | HZFGMQJYAFHESD-UHFFFAOYSA-M | | CAS DataBase Reference | 91714-93-1 |
| Hazard Codes | N | | Risk Statements | 50 | | Safety Statements | 61 | | RIDADR | UN 3077 9 / PGIII | | WGK Germany | 3 |
| | Bromfenac sodium Usage And Synthesis |
| Description | Bromfenac Sodium was launched in the US as a potent, orally-active, long lasting peripheral analgesic with antiinflammatory properties. It is structurally similar to ketoprofen and diclofenac and can be prepared in three steps from 2-amino-4’-bromophenone using Gassman’s oxindole synthesis. Duract’s biological effects are a result of its ability to reduce prostaglandin production through inhibition of cyclooxygenase. As a 4-bromo derivative of Amfenac, this modification increased the duration of analgesic activity and antiinflammatory potency. It was also free of any CNS, cardiovascular or autonomic effects. In comparison, 5 mg of Duract was equipotent to 650 mg of ASA and 25 mg was slightly more potent than 400 mg of Ibuprofen. | | Description | Bromfenac is an inhibitor of cyclooxygenase 2 (COX-2; IC50 = 6.6 nM) that is selective over COX-1 (IC50 = 210 nM). It inhibits prostaglandin E2 (PGE2; ) production in a rabbit model of LPS-induced eye inflammation. Bromfenac also decreases inflammation following cataract removal in dogs when administered as a 0.9% topical solution on a tapering schedule for 24 weeks. Formulations containing bromfenac have been used in the treatment of postoperative inflammation following cataract surgery. | | Originator | American Home Products (US) | | Uses | Bromfenac (Xibrom, ISTA Pharmaceuticals, Irvine, USA; Bronuck, Senju Pharmaceutical, Osaka, Japan) is indicated for the treatment of postoperative inflammation and the reduction of ocular pain in patients after undergoing cataract extraction. For this task, one drop of Xibrom may be applied to the affected eye twice daily beginning 24 hours after cataract surgery and continuing for the first 2 weeks of the postoperative period. The clinical safety and efficacy of bromfenac have been extensively studied in diverse comparative investigations, including the treatment of external or anterior ocular inflammatory diseases, allergic conjunctivitis, scleritis, and postoperative inflammation.The results of two phase III multicenter, randomized double-masked placebo-controlled clinical trials showed that bromfenac ophthalmic solution 0.09% was effective in the rapid resolution of ocular pain after cataract surgery, and there was a statistically significant difference between the bromfenac and placebo groups demonstrated in these phase III clinical trials. | | Uses | Bromfenac sodium has been used:
- to study its ability to bind to melanin
- in the synthesis of bromfenac indolinone standard
- to analyze its permeability in porcine conjunctiva
| | Definition | ChEBI: The sodium salt of bromfenac. Note that 'bromfenac sodium' commonly refers to the sesquihydrate (120638-55-3); this is the anhydrous form. | | Manufacturing Process | Reaction of (2-aminophenyl)-(4-bromophenyl)-methanone with
methylsulfanylacetic acid ethyl ester and tert-butyl hypochlorite gives a
corresponding sulfonium salt. This salt was transformed to initially to the
betaine. Electrocyclic rearrangement of that transient intermediate leads, after
rearomatization, to the homoanthranilic acid. Internal ester-amine interchange
leads then to 4-bromophenyl-(3-(methylthio)indolin-7-yl)methanone. The
thiomethyl group is then removed with Raney nickel to give 4-bromophenyl-
(indolin-7-yl)methanone. Saponification of this intermediate affords the (2-
amino-3-(4-bromobenzoyl)-phenyl)-acetic acid (Bromfenac).
In practice it is usually used as sodium salt. | | Brand name | Duract | | Therapeutic Function | Analgesicá Antiinflammatory | | Biochem/physiol Actions | Bromfenac exhibits antipyretic and prostaglandin synthetase inhibiting properties. It has therapeutic properties against the reduction of ocular pain and inflammation in postoperative cataract patients. Bromfenac acts as an effective agent against allergic conjunctivitis. It has the potential to treat acute muscle pain, osteoarthritis, and rheumatoid arthritis. |
| | Bromfenac sodium Preparation Products And Raw materials |
|