- Amitriptyline
-

- $0.00 / 100g
-
2023-09-14
- CAS:50-48-6
- Min. Order: 100g
- Purity: 99%
- Supply Ability: 20 tons
- Amitriptyline
-

- $30.00 / 1kg
-
2023-09-07
- CAS:50-48-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000t/year
- Amitriptyline
-

- $1.00 / 10g
-
2022-12-29
- CAS:50-48-6
- Min. Order: 10g
- Purity: 99.5%
- Supply Ability: 1000kg
|
| | Amitriptyline Basic information |
| Product Name: | Amitriptyline | | Synonyms: | 5-(3-Dimethylaminopropylidene)-10,11-dihydro-5H-dibenzo(a,d)cycloheptene;5-(3'-Dimethylaminopropylidene)-dibenzo-(a,d)(1,4)-cycloheptadiene;5-(3-Dimethylpropylidene)dibenzo(a,d)(1,4)cycloheptadiene;5-(gamma-Dimethylaminopropylidene)-10,11-dihydro-5H-dibenzo(a,d)cycloheptene;5-(gamma-Dimethylaminopropylidene)-5H-dibenzo(a,d)-10,11-dihydrocycloheptene;5-(gamma-Dimethylaminopropylidene)-5H-Dibenzo[a,d][1,4]cycloheptadiene;5-(gamma-Dimethylaminopropylidine)-5H-dibenzo(a,d)(1,4)cycloheptadiene;3-(10,11-dihydro-5h-dibenzo(a,d)cyclohepten-5-ylidene)-n,n-dimethyl-1-propan | | CAS: | 50-48-6 | | MF: | C20H23N | | MW: | 277.4 | | EINECS: | 200-041-6 | | Product Categories: | | | Mol File: | 50-48-6.mol | 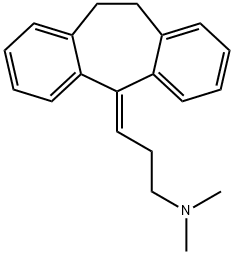 |
| | Amitriptyline Chemical Properties |
| Melting point | 196-197°C | | Boiling point | 410.26°C (rough estimate) | | density | 0.9415 (rough estimate) | | refractive index | 1.7500 (estimate) | | storage temp. | Store at -20°C | | form | Liquid | | pka | 9.4(at 25℃) | | color | Colorless to light yellow | | Water Solubility | 9.7 mg/mL | | Stability: | Light Sensitive | | CAS DataBase Reference | 50-48-6(CAS DataBase Reference) | | NIST Chemistry Reference | Amitriptyline(50-48-6) | | EPA Substance Registry System | Amitriptyline (50-48-6) |
| | Amitriptyline Usage And Synthesis |
| Originator | Elavil HCl Merck Sharp and,Dohme,US,1961 | | Uses | Amitriptyline is used for anxious-depressive conditions. It is easier to tolerate than
imipramine. | | Uses | Antidepressant. | | Definition | ChEBI: An organic tricyclic compound that is 10,11-dihydro-5H-dibenzo[a,d][7]annulene substituted by a 3-(dimethylamino)propylidene group at position 5. | | Manufacturing Process | Phthalic anhydride is reacted with phenylacetic acid to form 3-
benzylidenephthalide which is then hydrogenated to 2-phenethylbenzoic acid.
Conversion to the acid chloride followed by intramolecular dehydrochlorination
yields the ketone, 5H-dibenzo[a,d]cyclohepten-5-one. The ketone undergoes a
Grignard reaction with 3-(dimethylamino)propyl chloride to give 5-(γ-
dimethylaminopropylidene)-5H-dibenzo[a,d]cycloheptene.
Then, as described in US Patent 3,205,264, a solution of 5-(γ-
dimethylaminopropylidene)-5H-dibenzo[a,d]cycloheptene (42 grams; 0.153
mol) in 105 ml of ethanol is hydrogenated over Raney nickel (1.5 grams) at
65°C under an initial hydrogen pressure of 450 lb. After 1 mol of hydrogen is
absorbed (3.5 hours), the reaction mixture is filtered to remove the catalyst
and is acidified with 80 ml of 2.5 N hydrochloric acid (0.2 mol). The acidic
solution is concentrated to dryness under vacuum and is flushed three times
with 100 ml of benzene to remove residual water. The solid residue then is
dried under vacuum at 40°C to yield 44.9 grams (94% of theory) of the
product, MP 187-189.5°C, equivalent weight 307, ultraviolet absorption A%
2380432. Recrystallization from isopropyl alcohol and ether affords the product
in high purity.
In practice it is usually used as hydrochloride. | | Brand name | Elavil (AstraZeneca); Endep (Roche);Ami-anelun;Amilent;Amilit-ifi;Aminiurin;Amitimid;Amitriptol;Amyline;Amyzol;Annolytin;Apo-amitriptylline;Apo-pram;Deprelio;Deprestal;Diapatal;Elatrolet;Elavil plus;Emitrip;Enovil;Entrafon-210;Entrafon-2-10;Entrafon-2-25;Entrafon-a;Entrafon-forte;Etarfon;Etrafon-a;Etrafon-forte;Laroxal;Larozyl;Levate;Limbatarail;Limbatral;Limbitryl;Limitrol;Longopax;Loxaryl;Mareline;Meravil;Muaban d;Mutaban a/d/f;Nobrital;Novotriptyn;Novotryptin;Novo-tryptin;Parks-plus;Pms levazine;Prouvil;Saratem;Sarotena;Sedans;Sylvemid;Tensorelax;Teperin;Trepiline;Trepulin;Triptizol;Triptonal;Triptpane;Trivial-4-10. | | Therapeutic Function | Antidepressant | | World Health Organization (WHO) | Amitriptyline, a tricyclic antidepressant was introduced in 1961
for the management of endogenous depression and is listed in the 8th WHO Model
List of Essential Drugs. Much of the adverse effects are caused by its
antimuscarinic actions. These include dry mouth, cardiac arrhythmias, central
nervous system disturbances, blood disorders and risk of suicide. The risk of
suicide and dangers related to overdosage led the Norwegian Medicines Control
Authority to put the higher strength formulation under prescribing restriction in
1992. The risk of death following overdosage is apparently higher for products
containing tricyclic compounds as compared with nontricyclic products. | | Biological Functions | Amitriptyline is a tertiary amine dibenzocycloheptadiene TCA with a propylidene side chain extending from the central carbocyclic ring. The diarylpropylideneamine moiety for amitriptyline makes it sensitive to
photo-oxidation; therefore, its hydrochloride solutions should be protected from light to avoid ketone
formation and precipitation. | | Pharmacokinetics | Amitriptyline is rapidly absorbed from the GI tract and from parenteral sites.Amitriptyline and its active metabolite, nortriptyline, are distributed into breast milk.
Amitriptyline is primarily (65%) metabolized by N-demethylation by CYP2D6 to nortriptyline and hydroxylation
to its E-10-hydroxy metabolite. Nortriptyline is pharmacologically active as a secondary amine TCA.
Amitriptyline shows approximately equal affinity for 5-HT and NE transporters. | | Synthesis | Amitriptyline, 5-(3-dimethylaminopropyliden)-10,11-dihydrodibenzocy�cloheptene (7.1.4), differs from imipramine in that the nitrogen atom in the central part of the tricyclic system is replaced by a carbon, which is bound to a side chain by a double bond. Amitriptyline (7.1.4) is synthesized by interaction of 10,11-dihydro-N,N-dimethyl- 5H-dibenzo[a,d]cyclohepten-5-one with 3-dimethylaminopropylmagnesium bromide and the subsequent dehydration of the resulting tertiary alcohol (7.1.3) using hydrochloric acid [6–11].
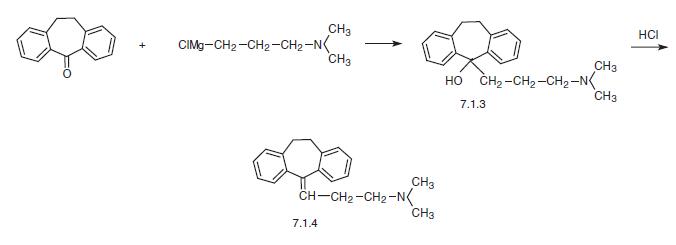
An alternative way of synthesis of amitriptyline is by interaction of 10,11-dihydro-N,N�dimethyl-5H-dibenzo[a,d]-cyclohepten-5-one with cyclopropylmagnesium bromide, giving 10,11-dihydro-N,N-dimethyl-5H-dibenzo[a,d]-cyclohepten-5-cyclopropyl-5-ol (7.1.5). Reacting this with hydrogen bromide in acetic acid results in an opening of the cyclopropyl ring, which forms 5-(3-bromopropyliden)-10,11-dihydro-5H-dibenzo[a,d]-cycloheptene (7.1.6). Alkylating this with dimethylamine gives amitriptyline (7.1.4) [12,13].
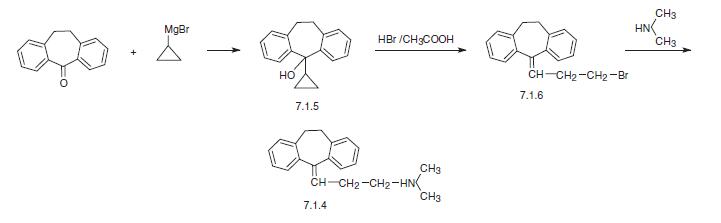 |
| | Amitriptyline Preparation Products And Raw materials |
|