- FERROCENE
-

- $9.00 / 10g
-
2024-04-26
- CAS:102-54-5
- Min. Order: 10g
- Purity: 99%
- Supply Ability: 10 tons
- Ferrocene
-

- $9.00 / 1kilograms
-
2024-04-26
- CAS:102-54-5
- Min. Order: 1kilograms
- Purity: 99%
- Supply Ability: 100tons
- Ferrocene
-

- $60.00 / 1kg
-
2024-04-02
- CAS:102-54-5
- Min. Order: 1kg
- Purity: 98.50%
- Supply Ability: 50MT/Month
|
| | Ferrocene Chemical Properties |
| Melting point | 172-174 °C (lit.) | | Boiling point | 249 °C (lit.) | | density | 1.490 | | vapor pressure | 0.03 mm Hg ( 40 °C) | | Fp | 100°C | | storage temp. | Store below +30°C. | | solubility | insoluble in H2O; soluble in ethanol, ethyl ether,benzene, dilute HNO
3 | | form | crystal | | color | orange | | Water Solubility | practically insoluble | | Sensitive | Air & Moisture Sensitive | | Sublimation | 100 ºC | | Merck | 14,4037 | | Exposure limits | ACGIH: TWA 10 mg/m3; TWA 1 mg/m3
OSHA: TWA 15 mg/m3; TWA 5 mg/m3
NIOSH: TWA 10 mg/m3; TWA 5 mg/m3; TWA 1 mg/m3 | | Stability: | Stable at room temperature. Incompatible with strong oxidizing agents. Highly flammable. | | LogP | 3.711 at 22℃ | | CAS DataBase Reference | 102-54-5(CAS DataBase Reference) | | NIST Chemistry Reference | Ferrocene(102-54-5) | | EPA Substance Registry System | Ferrocene (102-54-5) |
| | Ferrocene Usage And Synthesis |
| Organic transition metal | Ferrocene is an organic transition metal compound that has aromatic properties and is also known as dicyclopentadienyl iron. Its molecular structure contains a divalent iron cation and two cyclopentadienyl anions. It is used as a raw material in the production of ferrocenecarboxylic acid and was first successfully produced in the 1950s by reacting cyclopentadienyl magnesium bromide with anhydrous ferric chloride. Ferrocene is a non-polar compound that appears as an orange needle crystal powder with a camphor-like smell at room temperature. It is soluble in many organic solvents, including methanol, ethanol, ethyl ether, petroleum ether, gasoline, kerosene, diesel oil, methylene chloride, benzene, toluene, and xylene. Ferrocene has a molecule with polarity, high thermal stability, chemical stability, and radiation resistance, making it useful in industry, agriculture, medicine, aerospace, energy, environmental protection, and other fields. | | Chemical Properties | Ferrocene is a crystal with orange needle-like structure that sublimates at temperatures higher than 100℃ and melts at 172.5-173℃. Its boiling point is 249℃. It is soluble in dilute nitric acid, concentrated sulfuric acid, benzene, ether, petroleum ether and tetrahydrofuran and generates bluish fluorescence-containing deep red solution in dilute nitric acid and concentrated sulfuric acid. It is insoluble in water, 10% sodium hydroxide and hot concentrated hydrochloric acid, and it remains stable in air. Ferrocene has a strong property of UV absorbing and great thermostability, withstanding heating up to 470℃. It has a camphor-like scent and can be evaporated with water vapor. Ferrocene is neither dissolved nor decomposed in boiling solutions of these solvents. | | Uses | Ferrocene and its derivatives have various applications in different fields. It can be used as additives in rocket fuel, antiknock agent in gasoline, curing agent in rubber and silicone resin, and ultraviolet absorber. It can also be used in the production of metal-containing polymers and as a coating material for spacecraft. Ferrocene has a smoke abatement and combustion facilitating effect, which can reduce smoke production and increase power efficiency in fuel. It can also be used as an iron fertilizer for plants and as a pesticide. Ferrocene derivatives have applications as antioxidants, stabilizers, catalysts, and promoting agents in different industrial and organic synthesis processes. Addition of ferrocene to fuels such as diesel, gasoline, heavy oil, and coal can decrease the fuel consumption rate and reduce smoke production. Overall, ferrocene has unique characteristics that make it suitable for various applications in different fields. | | Category | toxic substances
| | Toxicity grading | poisoning
| | Acute toxicity | Oral-rat LD50: 1320 mg/kg; Oral-Mouse LD50: 832 mg/kg.
| | Flammability and hazard characteristics | flammable with the combustion generating iron-containing acrid smoke
| | Storage properties | warehouse: ventilated, low temperature and dry; Store it separately from oxidants.
| | Extinguishing agent | Water, carbon dioxide, dry, sandy soil.
| | Professional standards | TWA 10 mg/m³; STEL 20 mg/m3 | | Description | Ferrocene, a metallocene, is a bright orangesalt-like crystals from alcohol with a camphor odor.Molecular weight = 186.05; Boiling point = 249 C (sublimes); Freezing/Melting point = 173 C. Decomposes at465 C. Hazard Identification (based on NFPA-704 MRating System): Health 2, Flammability 2, Reactivity 1.Insoluble in water. | | Chemical Properties | Orange, crystalline solid; camphor-like
odor.
Insoluble in water; soluble in benzene, ether, and
alcohol. Iron content 29.4–30.6%. | | Chemical Properties | Ferrocene, a metallocene, is a bright orange
salt-like crystals from alcohol. Camphor odor. | | Physical properties | Orange crystals; camphor-like odor; melts at 172.5°C; vaporizes at 249°C; sublimes above 100°C; thermally stable above 500°C; insoluble in water; soluble in alcohol, ether and benzene; also soluble in dilute nitric acid and concentrated sulfuric acid forming a deep red solution that fluoresces. | | Uses | Ferrocene
is used as a catalyst for vulcanization, acceleration, and
polymerization, as a chemical intermediate for polymeric
compounds such as high temperature polymers, as an antiknock
additive for gasoline, as a coating for missiles and
satellites, and as a high-temperature lubricant. | | Uses | In ultraviolet stabilizers and smoke
depressants for polymers; to increase the burn
rate of rocket propellants; to prevent erosion of
space capsule shields; to improve the viscosity
of lubricants; to catalyze polymerization reactions;
to catalyze combustion; some derivatives
used as hematinic agents | | Uses | Antiknock additive for gasoline; catalyst. | | Preparation | Dicyclopentadienyliron may be obtained in a single-step synthetic route by heating cyclopentadiene with iron or iron pentacarbonyl at 300°C:
2C5H5 + Fe → (C5H5)2Fe
Also, it can be prepared by the reaction of iron(II) chloride with cyclopentadiene in the presence of an alkyl amine or a similar base.
Another convenient method of preparing this π-complex of iron is a twostep process in which the first step involves preparation of cyclopentadienyl Grignard reagent, such as 2,4-cyclopentadienylmagnesium bromide C5H5MgBr which may then be combined with ferric chloride to yield dicyclopentadienyl iron:
3C5H5MgBr + FeCl3 → (C5H5)2Fe + 3MgBrCl
Another general method of preparation involves the reaction of cyclopentadiene with sodium metal or sodium hydride in tetrahydrofuran (THF). Addition of iron(II) chloride to this solution forms the complex dicyclopentadienyliron:
2C5H6 + 2Na → 2C5H5ˉ + 2Na+ + H2
In 3:2 molar ratio of cyclopentadiene to sodium cyclopentene is obtained along with cyclopentadienidide (C5H5ˉ ) anion:
3C5H6 + 2Na → 2C5H5¯ + 2Na+ + C5H8
FeCl2 + 2C5H6Na → (C5H5)2Fe + 2NaCl | | Definition | A coordination compound of ferrous iron and two molecules of
cyclopentadiene in which the organic portions have
typically aromatic chemical properties. Its activity is intermediate between phenol and anisole.
The first compound shown to have the “sandwich | | Production Methods | Ferrocene is produced from the reaction of cyclopentadiene
with reduced iron in the presence of metal oxides. There is
also a two-stage production process in which produced iron
(II)oxide (stage 1) is reacted with cyclopentadiene. | | Definition | ferrocene: An orange-red crystallinesolid, Fe(C5H5)2; m.p. 173°C. Itcan be made by adding the ioniccompound Na+C5H5- (cyclopentadienylsodium, made from sodium andcyclopentadiene) to iron(III) chloride.In ferrocene, the two rings are parallel,with the iron ion sandwiched betweenthem (hence the namesandwich compound: see formula).The bonding is between pi orbitalson the rings and d-orbitals on theFe2+ ion. The compound can undergoelectrophilic substitution on theC5H5rings (they have some aromatic character).It can also be oxidized to theblue ion (C5H5)2Fe+. Ferrocene is the first of a class of similar complexescalled sandwich compounds. Its systematicname is di-π-cyclopentadienyliron(II). | | General Description | Orange crystalline solid or orange-yellow powder. Sublimes above 212°F. Camphor odor. | | Air & Water Reactions | Sensitive to prolonged exposure to air and may be sensitive to light. Insoluble in water. | | Reactivity Profile | Ferrocene reacts violently with tetranitromethane. . Contact of tetranitromethane with Ferrocene under various conditions leads to violent explosion, [Trans. Met. Chem., 1979, 4, 207-208]. | | Hazard | Moderate fire risk. Evolves toxic products
on decomposition and heating. | | Health Hazard | Dicyclopentadienyl iron causes
changes in blood parameters and hepatic
cirrhosis.
The toxicological properties of dicyclopentadienyl
iron have not been extensively
investigated. However, it has been used as a
preventive and therapeutic iron deficiency
drug, and its utilization is listed as tolerable. | | Fire Hazard | Flash point data for Ferrocene are not available. Ferrocene is probably combustible. | | Flammability and Explosibility | Highly flammable | | Safety Profile | Poison by
intraperitoneal and intravenous routes.
Moderately toxic by ingestion. Questionable
carcinogen with experimental tumorigenic
data. Mutation data reported. Flammable;
reacts violently with NH4ClO4. When heated
to decomposition it emits acrid smoke and
irritating fumes. | | Potential Exposure | Used as additive in fuel oil; antiknock
agent in gasoline fuel; used in making rubber, silicone
resins, high-temperature polymers and lubricants; interme diate for high-temperature polymers; as a smoke suppres sant and catalyst | | First aid | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. | | Carcinogenicity | Ferrocene was administered by
intramuscular injection at a dose of 5175 mg/kg/2 years.
By the criterion established by the Registry of Toxic Effects
of Chemical Substances (RTECS), ferrocene was an equivocal
tumorigenic agent and tumors were most evident at the
site of multiple injections. | | storage | Color Code—Red: Flammability Hazard: Store ina flammable materials storage area. Prior to working withthis chemical you should be trained on its proper handlingand storage. Store in tightly closed containers in a cool,well-ventilated area away from oxidizers, ammonium perchlorate, tetranitromethane, mercury(II) nitrate, and heat.Sources of ignition, such as smoking and open flames, areprohibited where this chemical is used, handled, or stored ina manner that could create a potential fire or explosionhazard. | | Shipping | UN1325 Flammable solids, organic, n.o.s.,
Hazard Class: 4.1; Labels: 4.1-Flammable solid. | | Purification Methods | Purify it by crystallisation from pentane or cyclohexane (also *C6H6 or MeOH can be used). It is moderately soluble in Et2O and sublimes readily above 100o. Crystallisation from EtOH gave material m 172.5-173o. [Wilkinson Org Synth Coll Vol IV 473 1963, Miller J Chem Soc 632 1952.] It has also been crystallised from methanol and sublimed in vacuo. [Saltiel et al. J Am Chem Soc 109 1209 1987, Beilstein 16 IV 1783.] | | Structure and conformation | X-ray diffraction studies show that in crystalline ferrocene (and in its
substituted derivatives) the iron atom is "sandwiched" between the two cyclopentadienyl
rings, these rings having the staggered configuration relative to each other (Fig. 16). The
rings are parallel plane regular pentagons, all the C-C and Fe-C distances being equal.
Electron diffraction studies show, however, that ferrocene has the eclipsed configuration
in the vapour state.
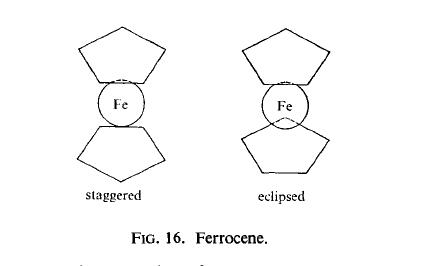 | | Incompatibilities | Violent reaction with ammonium per chlorate, tetranitromethane, mercury(II) nitrate. Incompa tible with oxidizers (chlorates, nitrates, peroxides, perman ganates, perchlorates, chlorine, bromine, fluorine, etc.);
contact may cause fires or explosions. Keep away from
alkaline materials, strong bases, strong acids, oxoacids,
epoxides.
Peroxomonosulfuric acid. Decomposes @≧465 ℃. |
| | Ferrocene Preparation Products And Raw materials |
| Raw materials | Sodium hydroxide-->Tetrahydrofuran-->Diethylamine-->Pentane-->1,3-Cyclopentadiene-->Ferrous chloride-->CYCLOHEXANE-D12-->ANHYDROUS FERRIC CHLORIDE | | Preparation Products | 1,1'-DIMETHYLFERROCENE-->1,1'-Bis (di-t-butylphosphino)ferrocene palladium dichloride,-->1,1'-Bis(di-tert-butylphosphino)ferrocene-->1,1'-Bis(diphenylphosphino)ferrocene-->1-DIPHENYLPHOSPHINO-1'-(DI-TERT-BUTYLPH&-->1 1'-BIS(DIPHENYLPHOSPHINO)FERROCENE-->1,1'-FERROCENEDICARBOXALDEHYDE-->1,1'-BIS(DIISOPROPYLPHOSPHINO)FERROCENE-->1,1'-DI-N-BUTYLFERROCENE-->Butylferrocene-->Benzoylferrocene-->1,1'-Diacetylferrocene-->dibuty cebacate with ferric chloride-->Chlorocarbonyl ferrocene-->1,1'-DIETHYLFERROCENE-->Methylcyclopentadienyl manganese tricarbonyl-->FERROCENEBORONIC ACID-->1,1'-binaphthalene-2,2'-diyl diacetate-->Acetylferrocene |
|