| Company Name: |
Leancare Ltd.
|
| Tel: |
+33 962096793 |
| Email: |
enquiry@leancare.co.uk |
| Products Intro: |
|
| Company Name: |
2A PharmaChem USA
|
| Tel: |
(630) 737-0988 |
| Email: |
sales@2apharmachem.com |
| Products Intro: |
|
|
| | Arnolol Basic information |
| Product Name: | Arnolol | | Synonyms: | Arnolol;3-Amino-1-[4-(2-methoxyethyl)phenoxy]-3-methylbutan-2-ol;1-[4-(2-methoxyethyl)-phenoxy]-3-amino-3-methyl-butan-2-ol;2-Butanol, 3-amino-1-[4-(2-methoxyethyl)phenoxy]-3-methyl- | | CAS: | 87129-71-3 | | MF: | C14H23NO3 | | MW: | 253.34 | | EINECS: | | | Product Categories: | | | Mol File: | 87129-71-3.mol | 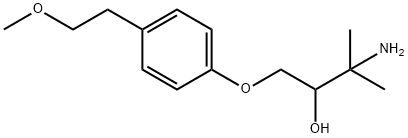 |
| | Arnolol Chemical Properties |
| | Arnolol Usage And Synthesis |
| Originator | Arnolol,ZYF Pharm Chemical | | Manufacturing Process | 2 methods of producing of 1-[4-(2-methoxyethyl)-phenoxy]-3-amino-3-
methyl-butan-2-ol:
1). 19.0 g of 1-[4-(2-methoxyethyl)phenoxy]-3-nitro-3-methylbutan-2-ol are
hydrogenated at 60°C and under a pressure of 6 bar in a mixture of 250 ml of
ethanol and 50 ml of glacial acetic acid in the presence of 4.0 g of palladiumon-
charcoal (10% Pd). After the mixture has been filtered and the solvent has
been evaporated off, the residue is rendered alkaline with 2 N NaOH and the mixture is extracted with CH2Cl2. The organic phase is washed with water and
dried with Na2SO4 to give, after evaporation, 15.0 g of the 1-[4-(2-
methoxyethyl)phenoxy]-3-amino-3-methylbutan-2-ol, melting point 120°-
122°C (dec.).
2). 18.3 g of zinc dust are gradually added to 20.0 g of 1-[4-(2-methoxyethyl)
phenoxy]-3-nitro-3-methylbutan-2-ol in 400 ml of ethanol and 60 ml of
concentrated hydrochloric acid at 50°-60°C. The mixture is stirred at this
temperature for 1 h and filtered and the solvent is evaporated off. After 200
ml of 40% strength NaOH have been added, the product is extracted with
methylene chloride. Solvent is distilled and the 1-[4-(2-methoxyethyl)
phenoxy]-3-amino-3-methylbutan-2-ol is produced, melting point 120°-122°C
(dec.). | | Therapeutic Function | Beta-adrenergic blocker (ophthalmic) |
| | Arnolol Preparation Products And Raw materials |
|