- Nitidine chloride
-

- $0.00 / 25kg
-
2024-04-12
- CAS:13063-04-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000ton
- Nitidine Chloride
-
- $0.00 / 20mg
-
2023-02-24
- CAS:13063-04-2
- Min. Order: 5mg
- Purity: ≥97%(HPLC)
- Supply Ability: 10 g
- Nitidine chloride
-

- $1.00 / 1kg
-
2019-07-06
- CAS:13063-04-2
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 100KG
|
| | Nitidine chloride Basic information |
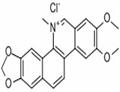
| Product Name: | Nitidine chloride | | Synonyms: | 2,3-Dimethoxy-12-methyl-(1,3)-benzodioxolo(5,6-c)phenanthridinium chloride;Nitidine chloride;[1,3]Dioxolo[4,5]benzo[1,2-c]phenanthridinium, 2,3-;dimethoxy-12-methyl-,chloride;NITIDINE;2,3-DiMethoxy-12-Methyl-[1,3]dioxolo[4',5':4,5]benzo[1,2-c]phenanthridin-12-iuM chloride;Angolinine;NITIDINE CHLORIDE (RG) | | CAS: | 13063-04-2 | | MF: | C21H18NO4+ | | MW: | 348.37 | | EINECS: | 683-192-4 | | Product Categories: | Active Pharmaceutical Ingredients;chemical reagent;pharmaceutical intermediate;phytochemical;reference standards from Chinese medicinal herbs (TCM).;standardized herbal extract | | Mol File: | 13063-04-2.mol |  |
| | Nitidine chloride Chemical Properties |
| Melting point | 283-286 °C | | storage temp. | -20°C | | solubility | DMSO: soluble1mg/mL, clear (warmed) | | form | powder | | color | white to beige | | InChIKey | QLDAACVSUMUMOR-UHFFFAOYSA-M |
| Safety Statements | 24/25 | | WGK Germany | 3 | | RTECS | DF4935500 | | HS Code | 29399990 | | Toxicity | mouse,LD50,intraperitoneal,98980ug/kg (98.98mg/kg),National Cancer Institute Screening Program Data Summary, Developmental Therapeutics Program. Vol. JAN1986, |
| | Nitidine chloride Usage And Synthesis |
| Description | This quaternary alkaloid occurs primarily in Zanthoxylum nitidum (Lam.) DC
but has also been found in several species of Fagara. It is normally isolated as the
chloride which forms bright yellow needles when recrystallized from EtOH-HCl.
The crystals decompose on heating to 240°C yielding a product having m.p.
285-6°C. The iodide also forms yellow needles and undergoes a similar trans_x0002_formation at the same temperature to yield a product with the same melting point, possibly identical to that obtained from the chloride. The crystalline
acetate, m.p. 255-260°C and the pseudocyanide, m.p. 215-6°C and decompos�ing completely at 234°C in vacuo have also been prepared. A periodide, which
fonns chocolate-brown needles when crystallized from Me2CO, m.p. 300-1 °c
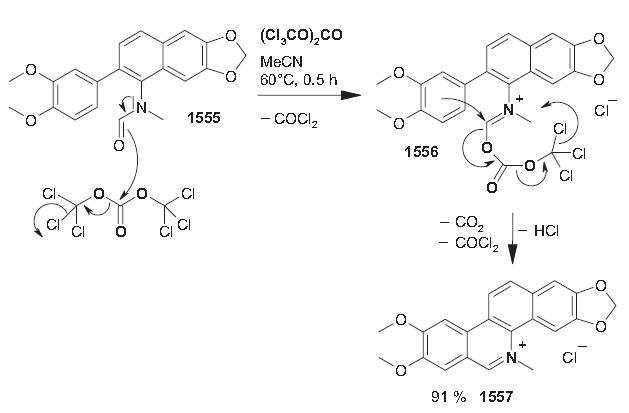
(dec.) is useful for characterizing the base. | | Uses | Nitidine Chloride shows the ability to inhibit hepatic cancer growth through modulation of signaling pathways. | | Preparation | A solution of 1555 (0.102 g, 0.278 mmol) and triphosgene (0.179 g, 0.602 mmol) in acetonitrile (2.5 mL) was stirred at 60 C° (bath temperature) for 0.5 h. After the addition of ice/water, a yellow precipitate was collected by filtration and recrystallized from ethanol/diethyl ether to directly afford nitidine chloride (0.098 g, 91%); mp 285–292 C°.
An isocyano group can serve as both a protecting group for the amino function, and, due to its electronic effect, as an activating group as well. These two functionalities are employed in a synthetic route whereby an amino function has to be protected and a condensation reaction is performed at the a-carbon atom, for which activation is required.
3,4-Fused tryptophan analogues 1563 and 1564 contain a ring that bridges the a-carbon and the 4-position of the indole ring, thus limiting the conformational flexibility of the side chain. The synthesis proceeds from N-formylated 40-bromotryptophan 1558 via isocyanide 1559, 2-propenoate 1560, and Pd-catalyzed cyclization of the a-2-propenyl dl-tryptophan derivatives 1561 and 1562 to give both the seven- and eight-membered constrained ring analogues 1564 and 1563. Dehydration of the formamide 1558 with triphosgene affords the isocyanide 1559 in 75% (87%) yield. | | Biochem/physiol Actions | Nitidine chloride is a natural product with anti-cancer activity. Its mechanism of action likely involves several pathways. Nitidine chloride has been found to inhibit topoisomerase I and topoisomerase II, induce cell apoptosis by activation of the caspase-dependent pathway, suppress c-Src/FAK associated signaling pathways and suppress Janus kinase 2/STAT3 signaling and the expression of STAT3-dependent target genes, including cyclin D1, Bcl-xL, and VEGF. Nitidine chloride has also been found to have anti-malaria activity. | | References | Arthur, Hui, Ng., Chern. Ind., 1514 (1958)
Arthur, Hui, Ng.,J. Chern. Soc., 1840 (1959)
Kuck, Albonico, Deulofeu., Chern. Ind., 945 (1966) |
| | Nitidine chloride Preparation Products And Raw materials |
|