- latamoxefsodium
-

- $0.00 / 1kg
-
2024-04-29
- CAS:64953-12-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20tons
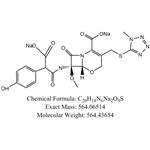
- Latamoxef Sodium
-
- $0.00 / 10mg
-
2024-04-10
- CAS:64953-12-4
- Min. Order: 10mg
- Purity: 90%+
- Supply Ability: 10g
- Latamoxef sodium
-

- $0.00 / 1Kg
-
2020-05-25
- CAS:64953-12-4
- Min. Order: 1KG
- Purity: 99.0%+
- Supply Ability: 300 MT
|
| | Latamoxef sodium Basic information |
| | Latamoxef sodium Chemical Properties |
| Melting point | >156oC (dec.) | | alpha | D22 -45° (water) | | storage temp. | Inert atmosphere,2-8°C | | solubility | Methanol (Slightly) | | form | White to Pale Yellow | | color | White to off-white | | Merck | 13,6311 | | Stability: | Unstable in solution (DMSO or Methanol) | | InChIKey | GRIXGZQULWMCLU-GDUWRUPCSA-L |
| Hazard Codes | Xn | | Risk Statements | 42/43 | | Safety Statements | 22-36/37-45 | | WGK Germany | 2 | | RTECS | RN6824000 |
| | Latamoxef sodium Usage And Synthesis |
| Description | Latamoxef was synthesized by Shionogi Pharmaceuticals in 1975 starting with benzylpenicillin and using a novel drug design. The oxacephem nucleus, in which the sulfur atom had been replaced by oxygen, was substituted with a methoxyl group at the 7α position, as in the cephamycins. A carboxyl moiety and a hydroxybenzyl group were added at the 7β position, as in carbenicillin, and a methyltetrazolylthiomethyl group was attached at the 3 position. These substitutions resulted in a strong activity against gram-negative bacteria and a high resistance to the action of βlactamase, along with excellent activity against Pseudomonas aeruginosa, even though the compound has no activity against Staphylococcus aureus. | | Originator | Moxam,Lilly,US,1981 | | Uses | Moxalactam Sodium is an oxacephem antibiotic. Moxalactam Sodium is more effective against Escherichia coli and Pseudomonas aeruginosa than cephalosporins. | | Uses | Antibacterial;Bacterial transpeptidase inhibitor | | Manufacturing Process | To a stirred suspension of p-(p-methoxybenzyloxy)-phenylmalonic acid (125
mg) in methylene chloride (3 ml) are added triethylamine (55 l) and oxalyl
chloride (26 l) at -15°C, and the suspension is stirred for 40 minutes at 0°C.
The mixture is added to a solution of diphenylmethyl 7β-amino-7α-methoxy-3-
(1-methyltetrazol-5-yl)thiomethyl-1-oxadethia-3-cephem-4-carboxylate (100
mg) in methylene chloride (3 ml) and pyridine (63 l), and the mixture is
stirred for 30 minutes at 0°C. The reaction mixture is diluted with ethyl
acetate, washed with aqueous 2N-hydrochloric acid and water, dried over
sodium sulfate, and concentrated to give crude product (212 mg), which is
chromatographed on silica gel (20 g) and eluted with a mixture of ethyl
acetate and acetic acid (99:1) to give diphenylmethyl-7β-[α-p-(p�methoxybenzyloxy)phenyl-α-carboxyacetamido]-7α-methoxy-3-(1-
methyltetrazol-5yl)thiomethyl-1-oxadethia-3-cephem-4-carboxylate as foam
(71 mg). Yield: 45%.
To a solution of diphenylmethyl-7β-[α-p-(p-methoxybenzyl)-oxy-phenyl-α-p�methoxybenzyl-oxycarbonil-acetamido]-7α-methoxy-3-(1-methyltetrazol-5-
yl)thiomethyl-1-oxadethia-3-cephem-4-carboxylate (1.20 g) in methylene
chloride (24 ml) are added anisole (2.4 ml) and a solution of aluminum
chloride (2.58 g) in nitromethane (12 ml) at 0°C under nitrogen. After stirring
for 15 minutes at 0°C, the mixture is poured into cold 5% sodium hydrogen
carbonate aqueous solution (100 ml) and filtered to remove the formed
precipitate. The filtate is washed twice with methylene chloride (2 x 100 ml),
acidified with 2N-hydrochloric acid to pH 2.60, and poured in a column of high
porous polymer HP-20 (60 ml) sold by Mitsubishi Chemical Industries Ltd. The
column is washed with water (300 ml) and eluted with methanol. The eluate
is concentrated under reduced pressure at room temperature. The residue is
dissolved in methanol, treated with active carbon, and concentrated under
reduced pressure to give 7β(α-p-hydroxyphenyl-α-carboxyacetamido)-7β-
methoxy-3-(1-methyl-tetrazol-5-yl)thiomethyl1-oxadethia-3-cephem-4-
carboxylic acid as powder (595 mg) decomposing at 125°C to 132°C. Yield:
88.5%.
To a solution of 7β(α-p-hydroxyphenyl-α-carboxyacetamido)-7α-methoxy-3-(1-
methyl-tetrazol-5-yl)thiomethyl1-oxadethia-3-cephem-4-carboxylic acid (359
mg) in methanol (7 ml) is added a solution of sodium 2-ethylhexanoate in
methanol (2 mols/liter; 1.73 ml) at room temperature. After stirring for 10
minutes, the reaction mixture is diluted with ethyl acetate, stirred for 5
minutes, and filtered to collect separated solid, which is washed with ethyl
acetate, and dried to give disodium salt of 7β(α-p-hydroxyphenyl-α-
carboxyacetamido)-7α-methoxy-3-(1-methyl-tetrazol-5-yl)thiomethyl1-
oxadethia-3-cephem-4-carboxylic acid (342 mg). Yield: 888%. Colorless
powder. MP decomposition from 170°C. | | Brand name | Moxam (Lilly). | | Therapeutic Function | Antiinfective | | Biological Activity | moxalactam (sodium salt) is a β-lactam antibiotic.β-lactam antibiotics (beta-lactam antibiotics) are a class of broad-spectrum antibiotics that contain a beta-lactam ring in their molecular structures. most β-lactam antibiotics act by inhibiting cell wall biosynthesis.moxalactam is an oxacephem antibiotic and inhibits most commonly occurring gram positive, gram negative, and anaerobic bacteria. moxalactam was more active against klebsiella and e coli than cefoperazone. moxalactam did not inhibit methicillin-resistant s. aureus and enterococci. moxalactam had been extremely active against haemophilis and neisseria gonorrhoeae. moxalactam was extremely resistant to hydrolysis by all plasmid and chromosmal β-lactamases [1]. in certain situations, moxalactam impaired normal hemostasis and prolonged bleeding time [2]. | | references | [1]. neu hc. the in vitro activity, human pharmacology, and clinical effectiveness of new beta-lactam antibiotics. annu rev pharmacol toxicol. 1982;22:599-642.
[2]. sattler fr, weitekamp mr, ballard jo. potential for bleeding with the new beta-lactam antibiotics. ann intern med. 1986 dec;105(6):924-31. |
| | Latamoxef sodium Preparation Products And Raw materials |
|