- Metolazone
-

- $15.00 / 1KG
-
2021-07-13
- CAS:17560-51-9
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Metolazone
-

- $15.00 / 1KG
-
2021-07-09
- CAS:17560-51-9
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Metolazone
-

- $1.00 / 1KG
-
2020-01-09
- CAS:17560-51-9
- Min. Order: 1KG
- Purity: 98% HPLC
- Supply Ability: 10 tons/month
|
| | Metolazone Basic information |
| Product Name: | Metolazone | | Synonyms: | s720-22;7-Chloro-1,2,3,4-tetrahydro-2-methyl-3-(2-methylphenyl)-4-oxo-6-quinazolinesulfonamide, SR-720-22, Diulo, Metenix, Mykrox, Oldren,;6-Quinazolinesulfonamide, 7-chloro-1,2,3,4-tetrahydro-2-methyl-3-(2-methylphenyl)-4-oxo- (9CI);6-Quinazolinesulfonamide, 7-chloro-1,2,3,4-tetrahydro-2-methyl-4-oxo-3-o-tolyl- (8CI);Normelan;Xuret;6-Quinazolinesulfonamide, 7-chloro-1,2,3,4-tetrahydro- 2-methyl-3-(2-methylphenyl)-4-oxo-;METAZOLINE | | CAS: | 17560-51-9 | | MF: | C16H16ClN3O3S | | MW: | 365.83 | | EINECS: | 241-539-3 | | Product Categories: | ZAROXOLYN;Other APIs;Organics;Amines;Aromatics;Heterocycles;Sulfur & Selenium Compounds;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 17560-51-9.mol | 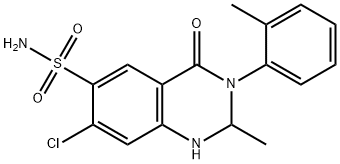 |
| | Metolazone Chemical Properties |
| Melting point | 252-254°C | | Boiling point | 613.6±65.0 °C(Predicted) | | density | 1.2895 (rough estimate) | | refractive index | 1.6100 (estimate) | | storage temp. | 2-8°C | | solubility | DMSO: >5mg/mL | | pka | pKa 9.7 (Uncertain) | | form | solid | | color | white | | Water Solubility | 60.3mg/L(25 ºC) | | Stability: | Stable for 2 years as supplied. Solutions in DMSO may be stored desiccated at -20°C for up to 2 months. | | InChIKey | AQCHWTWZEMGIFD-UHFFFAOYSA-N | | CAS DataBase Reference | 17560-51-9(CAS DataBase Reference) | | NIST Chemistry Reference | Metolazone(17560-51-9) |
| | Metolazone Usage And Synthesis |
| Description | Metolazone (Item No. 26304) is an analytical reference standard categorized as a diuretic. It has been detected as an adverse analytical finding (AAF) during anti-doping testing. This product is intended for use in analytical forensic applications. This product is also available as a general research tool . | | Chemical Properties | Crystalline Solid | | Originator | Zaroxolyn,Pennwalt,UK,1973 | | Uses | diuretic, antihypertensive | | Uses | Metolazone acts on the distal tubules, thus increasing excretion of water and sodium,
potassium, and chloride ions. It is used for treating edema caused by cardiac insufficiency
and adrenal irregularities, including nephrotic syndrome. | | Uses | A diruetic. An antihypertensive | | Definition | ChEBI: A quinazoline that consists of 1,2,3,4-tetrahydroquinazolin-4-one bearing additional methyl, 2-tolyl, sulfamyl and chloro substituents at positions 2, 3, 6 and 7 respectively. A quinazoline diuretic, with properties similar to thiazide diuretics. | | Manufacturing Process | Preparation of Intermediate Compound N-Acetyl-5-Chloro-2-Methylaniline: To
a well-stirred mixture of 1,270 g (9 mols) of 5-chloro-2-methylaniline in 7.5
liters of water at 34°C was added all at once 1,710 ml (18 mols) of acetic
anhydride. A solution was obtained and then almost immediately the product
started to crystallize. The temperature rose to 60°C. The mixture was stirred
until the temperature dropped to 30°C. The product was filtered and washed
well with water. Yield 97% (1,640 g), MP 134° to 138°C. Product was air dried
and then in vacuum over P2O5.
Preparation of Intermediate Compound 5-Chloro-2-Methyl-4-
Sulfamylacetanilide: Into a 3-necked 3-liter flask fitted with stirrer and
thermometer 540 ml of chlorosulfonic acid were placed and cooled in an ice
bath to 20°C. 300 g of the acetanilide were added portionwise while stirring
and maintaining temperature at 20°C. This addition takes approximately 20
minutes. Remove the ice bath and add 88 g of sodium chloride portionwise
(approximately 1 tsp every 10 minutes), This addition takes approximately 1
hour. Some foaming takes place. Using heating mantle bring temperature up
slowly (approximately ? hour) to 75°C. Considerable foaming takes place and
heating is continued another ? hour until 92°C is reached. Foaming can be
controlled by shutting off heat and with good stirring. Once the temperature
of 92°C has been reached and foaming has subsided reaction can be left
unattended. Keep reaction at 92°C for a total of 2? hours.
Pour the hot reaction mixture onto 4 liters of crushed ice. Pour slowly and stir
the ice mixture. What remains in the flask can be worked up by adding ice to
it and swirling the contents. After approximately 3/4 of an hour, the solid is
filtered and washed with approximately 600 ml water.
Break up cake into small pieces and add to 2.5 liters concentrated NH4OH in 4
liter beaker. Stir. Solid goes into solution and then the sulfonamide
precipitates out. Heat to 50°C and then turn off heat. After ? hour cool in ice
bath and filter. Wash cake with 600 ml water. Add cake to 2 liters 5% NaOH
(130 ml 50% NaOH to 2 liters water). Filter and discard insolubles. While
cooling filtrate add concentrated HCl until mixture is acid. Filter and wash cake
until filtrate is neutral. Suck cake as dry as possible then air dry. Yield
approximately 200 g (45%), MP 255° to 260°C.
Preparation of Intermediate Compound 4-Chloro-5-Sulfamyl-N�Acetylanthranilic Acid: To a hot solution (80°C) of 366 g (1.482 mols) of
magnesium sulfate (Epsom salts) in 2.8 liters of water was added 130 g
(0.495 mol) of powdered 5-chloro-2-methyl-4-sulfamylacetanilide. With
stirring and maintaining the temperature at 83°C, 234 g (1.482 mols) of
potassium permanganate was added portionwise over a period of 2 hours. The
mixture was then kept at 85°C with stirring for an additional 3 hours. By thistime the pink color of the permanganate had been discharged.
The mixture was cooled to 65°C and 250 g (2.0 mols) of sodium carbonate
monohydrate was added. The warm reaction mixture was filtered and the cake
washed with water. The filtrate was then slowly treated with concentrated
hydrochloric acid until mixture tested acid. Product was then filtered, washed
with water and dried. Yield 103 g (71.0%), MP 245° to 249°C (dec.).
Preparation of Intermediate Compound 2-Methyl-3-o-Tolyl-6-Sulfamyl-7-
Chloro-4(3H)-Quinazolinone: Set up a 5-liter 3-necked flask fitted with a
stirrer, condenser and a drying tube. To a stirred mixture of 100 g (0.342 mol)
of powdered 4-chloro-5-sulfamyl-N-acetylanthranilic acid, 40.2 g (0.376 mol)
of o-toluidine and 2.0 liters of dry toluene was added dropwise, over a period
of 15 minutes, 21.7 ml (34.1 g) (0.248 mol) of phosphorus trichloride. The
mixture was then refluxed for 10 hours. The solid turned somewhat gummy
towards the latter part of the first hour. The mixture then became more free
flowing as heating was continued. Let stand overnight. The yellow solid was
filtered, washed with toluene and dried. The toluene filtrate was discarded.
The dried solid was triturated with 1.5 liters of 10% sodium bicarbonate,
filtered and the cake washed with water. The filtrate on acidification yielded
11.5 g of the starting acid. The damp product was dissolved in 4.5 liters of
95% ethanol and the solution treated with charcoal and filtered. On cooling
filtrate yielded 69.5 g (55.5%) of the title compound, MP 271.5° to 274°C.
Preparation of the Final Compound 2-Methyl-3-o-Tolyl-6-Sulfamyl-7-Chloro-
1,2,3,4-Tetrahydro-4(3H)-Quinazolinone: To 4 liters of dry diglyme in a 12-
liter 3-necked flask fitted with a stirrer, thermometer and drying tube was
added 5.34 g (0.04 mol) of aluminum chloride, while stirring. To the resulting
solution was added 43.6 g (0.12 mol) of 2-methyl-3-o-tolyl-6-sulfamyl-7-
chloro-4(3H)-quinazoline. A solution of 4.56 g (0.12 mol) of sodium
borohydride in 1 liter of dry diglyme was added portionwise over a period of 1
hour while stirring the mixture. The mixture was then heated at 85°C, with
stirring, for 1 hour.
After cooling the reaction mixture to 25°C in an ice bath 600 ml of water was
added and then enough dilute hydrochloric acid (approximately 100 ml) to
make the solution acid. The solvent was then removed under reduced
pressure at 60° to 70°C. The very viscid residue solidified when triturated
with water. The solid was filtered and washed with water. The solid was
dissolved in approximately 400 ml 95% ethanol and the solution filtered
through Celite. On cooling the solution yielded 30 g of colorless solid, MP 253°
to 259°C. The filtrate was concentrated to 200 ml to yield another 4.6 g, MP
253° to 259°C.
The above product was then recrystallized from 900 ml of 95% ethanol after
filtering the hot solution through Celite. Crystallization was initiated and the
mixture agitated occasionally while being cooled in the refrigerator. Yield of
product 29 g, MP 253° to 259°C. Concentration of the filtrate to 125 ml
yielded another 7.5 g of product, MP 253° to 259°C. The product was
recrystallized another time in the manner described above. Total yield, first
and second crops, 28.8 g (66%), MP 250° to 255°C. Product was dried at
80°C in a vacuum, according to US Patent 3,360,518. | | Brand name | Diulo (Searle); Mykrox
(UCB); Zaroxolyn(UCB). | | Therapeutic Function | Diuretic | | Biochem/physiol Actions | Inhibitor of thiazide-sensitive Na+-Cl- cotransporter; antihypertensive; moderate "loop" diuretic. | | Synthesis | Metolazone, 7-chloro-1,2,3,4-tetrahydro-2-methyl-4-oxo-3-o-tolyl-6-quinazolinsulfonamide
(21.3.20), is synthesized from 5-chloro-2-methylaniline. The amino group
is acylated by ethyl chloroformate, forming 5-chloro-N-ethoxycarbonyl-2-methylaniline
(21.3.15). The product, upon subsequent reaction with chlorosulfonic acid and ammonia, is
transformed in the usual manner into 4-sulfonamido-5-chloro-N-ethoxycarbonyl-2-methylaniline
(21.3.16). The methyl group of this product is oxidized by potassium permanganate,
giving 5-sulfonamido-4-chloro-N-ethoxycarbonyl anthranylic acid (21.3.17). Upon treating
this with thionyl chloride it cycles into the corresponding anhydride (21.3.18). This reacts with
o-toluidine, turning it into 2-amino-5-aminosulfonyl-4-chloro-o-toluolbenzamide (21.3.19).
Finally, reacting this with dimethylacetal acetic acid gives metolazone (21.3.20). 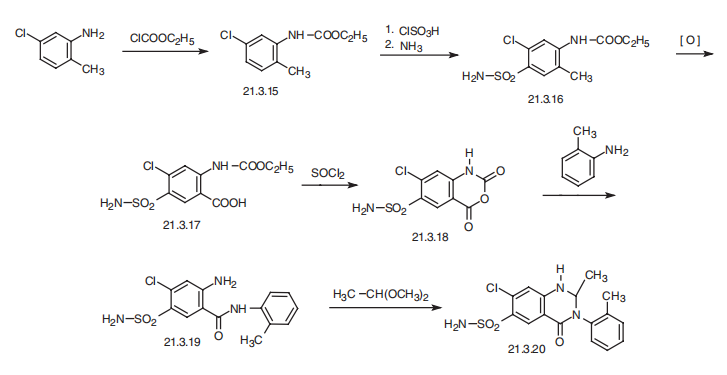
| | References | Ito et al. (2021), Metolazone upregulates mitochondrial chaperones and extends lifespan in Caenorhabditis elegans; Biogerontology, 22 119
Beaumont et al. (1988), Thiazide diuretic drug receptors in rat kidney: identification with [3H] metolazone; Proc. Natl. Acad. Sci. USA, 85 2311
Moreno et al. (2006), Affinity-defining domains in the Na-Cl cotransporter: a different location for Cl- and thiazide binding; J. Biol. Chem., 281 17266
Temperini et al. (2008), Carbonic anhydrase inhibitors. Interaction of indapamide and related diuretics with 12 mammalian isozymes and X-ray crystallographic studies for the indapamide-isozyme II adduct; Bioorg. Med. Chem. Lett., 18 2567 |
| | Metolazone Preparation Products And Raw materials |
|