- Potassium Iodate
-

- $10.00 / 1kg
-
2024-04-30
- CAS:7758-05-6
- Min. Order: 1kg
- Purity: 99.5
- Supply Ability: 10 ton per month
- Potassium iodate
-

- $50.00 / 10kg
-
2024-04-30
- CAS:7758-05-6
- Min. Order: 10kg
- Purity: 99.99%
- Supply Ability: 100Tons
- Potassium iodate
-

- $380.00 / 1kg
-
2023-11-29
- CAS:7758-05-6
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 1000000tons
|
| | Potassium iodate Basic information |
| Product Name: | Potassium iodate | | Synonyms: | POTASSIUM IODATE, REAGENTPLUS, 99.99+%;POTASSIUM IODATE, CERTIFIED STANDARD TIT RIM. SUBSTANCE;1/60 MOL POTASSIUM IODATE FIXANAL;POTASSIUM IODATE, 99.5%, A.C.S. REAGENT;POTASSIUM IODATE, 99.998%;0,05 mol/L Potassium Iodate;POTASSIUM IODATE R. G., REAG. ACS, REAG. ISO, REAG. PH. EUR.;POTASSIUM IODATE, VOLUMETRIC STANDARD, 0 .1N SOLUTION IN WATER | | CAS: | 7758-05-6 | | MF: | IKO3 | | MW: | 214 | | EINECS: | 231-831-9 | | Product Categories: | Pyridines;oxidant analytical reagent;N - RSynthetic Reagents;Salt Solutions;Titration;Volumetric Solutions;ACS GradeSynthetic Reagents;Essential Chemicals;Inorganic Salts;Potassium;Routine Reagents;Reference Material Sodium thiosulfateTitration;By Reference Material;Concentrates (e.g. FIXANAL);Salt Concentrates;Materials Science;Metal and Ceramic Science;Potassium Salts;Reagent Grade;O-P, Puriss p.a. ACS;Puriss p.a. ACS;Synthetic Reagents;Analytical Reagents for General Use;O-P, Puriss p.a.;Puriss p.a.;N - R;PON - PTCertified Reference Materials (CRMs);Titrimetry;Titrimetry CRMs;TitrimetryAnalytical Standards;Alphabetic;Application CRMs;P;Metal and Ceramic Science;Potassium Salts;Salts;Halogenated SolutionsVolumetric Solutions;Inorganics;7758-05-6 | | Mol File: | 7758-05-6.mol | 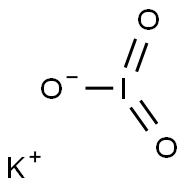 |
| | Potassium iodate Chemical Properties |
| Melting point | 560 °C(lit.) | | density | 3.93 g/mL at 25 °C(lit.) | | vapor pressure | 0-0Pa at 25℃ | | storage temp. | Store at +5°C to +30°C. | | solubility | H2O: 0.1 M at 20 °C, clear, colorless | | pka | 0.047[at 20 ℃] | | form | Powder/Solid | | color | White to off-white | | Odor | at 100.00?%. odorless | | PH | 6 (50g/l, H2O, 20℃) | | Water Solubility | Soluble | | Merck | 14,7642 | | Solubility Product Constant (Ksp) | pKsp: 3.43 | | Stability: | Stable. Materials to avoid include organics, combustibles, phosphorus, sulfur, carbon, powdered metals, cyanides, hydrides, strong reducing agents, aluminium, peroxides. Explosive when mixed with combustible material. | | InChIKey | JLKDVMWYMMLWTI-UHFFFAOYSA-M | | LogP | -1 at 25℃ | | CAS DataBase Reference | 7758-05-6(CAS DataBase Reference) | | EPA Substance Registry System | Potassium iodate (7758-05-6) |
| | Potassium iodate Usage And Synthesis |
| Description | Potassium iodate is an iodine-rich salt with the formula KIO3. In hot and humid climates, iodine vapor is hydrolyzed due to the hygroscopicity of potassium iodide. Compared to potassium iodide, potassium iodate is more stable and has a longer shelf life. It is a potent blocker of radioactive iodine uptake by the thyroid gland. | | Chemical Properties | white crystals or powder | | Physical properties | Colorless crystals or white powder; monoclinic structure; density 3.90 g/cm3; stable at ordinary temperatures; melts at 560°C with partial decompo-sition, releasing oxygen; moderately soluble in cold water; 4.74 g/100mL at 0°C; greater solubility in boiling water 32.3 g/100mL at 100°C; soluble in potassium iodide solution; insoluble in alcohol and liquid ammonia. | | Uses | Oxidizing agent in volumetric chemical analysis; as maturing agent and dough conditioner. | | Uses | Oxidizer used recently to form 2-styrylchromones from o-hydroxy-ω-cinnamylideneacetophenones by oxidative cyclization.1 | | Uses | Potassium Iodate is a source of iodine made by reacting iodine with
potassium hydroxide. it is a crystalline powder which is more stable
than iodide. it has a solubility of 1 g in 15 ml of water. it is used as
a fast-acting dough improver; it is used with potassium bromate as
an oxidizing agent to modify the protein in bread flour which pro-
motes loaf volume and shape. it is used in baked goods. | | Definition | A
white solid formed either by adding iodine
to a hot concentrated solution of potassium
hydroxide or by the electrolysis of
potassium iodide solution. No hydrates are
known. It is a source of iodide and iodic
acid. When treated with a dilute acid and a
reducing agent, the iodate ions are reduced
to iodine. | | Production Methods | Potassium iodate is formed (1) by electrolysis of potassium iodide under proper conditions, (2) by reaction of iodine and potassium hydroxide solution, and the fractional crystallization of iodate from iodide. Used as a source of iodate and iodic acid. | | Definition | potassium iodate: A white crystallinesolid, KIO3, soluble in waterand insoluble in ethanol; monoclinic;r.d. 3.9; m.p. 560°C. It may be preparedby the reaction of iodine withhot concentrated potassium hydroxideor by careful electrolysis ofpotassium iodide solution. It is anoxidizing agent and is used as ananalytical reagent. Some potassiumiodate is used as a food additive. | | Preparation | Potassium iodate can be produced by fusing potassium iodide with potassium chlorate, bromate or perchlorate:
KI + KClO3→KIO3+ KCl
The melt is extracted with water and potassium iodate is isolated from solution by crystallization. | | Application | Potassium iodate is a fairly strong oxidizing agent that may be used in the assay of a number of pharmaceutical substances, for instance : benzalkonium chloride, cetrimide, hydralazine hydrochloride, potassium iodide, phenylhydrazine hydrochloride, semicarbazide hydrochloride and the like. Under appropriate experimental parameters the iodate reacts quantitatively with both iodides and iodine. It is, however, interesting to observe here that the iodate titrations may be carried out effectively in the presence of saturated organic acids, alcohol and a host of other organic substances.
The oxidation-reduction methods with potassium iodate invariably based on the formation of iodine monochloride (ICl) in a medium of strong hydrochloric acid solution. | | General Description | KIO3 can be used as a substitute of KI in radiation protection. A kinetic study of thermal degradation of KIO3 by γ-rays suggests that rate of decomposition increases while activation energy decreases upon irradiation. | | Flammability and Explosibility | Non flammable | | Food additive | Iodine can be added to salt in the form of potassium iodide (KI) or potassium iodate (KIO3). Because KIO3 has higher stability in the presence of salt impurities, humidity, and porous packaging, it is the recommended form.
Potassium iodate, which is formed as a secondary product, is reduced by activated carbon. The product is purified by crystallization from water. Alternatively, iron (II) iodide, prepared by using iron powder and iodine, can be treated with potassium carbonate to obtain potassium iodide. High-purity potassium iodide can be prepared by the reaction of a potassium bicarbonate with hydriodic acid. | | Safety Profile | Poison by ingestion and
intraperitoneal routes. A trace mineral added
to animal feeds. Potentially explosive
reaction with charcoal + ozone, metals (e.g.,
powdered aluminum, copper), arsenic
carbon, phosphorus, sulfur, alkali metal
hydrides, alkaline earth metal hydrides,
antimony sulfide, arsenic sulfide, copper
sulfide, tin sulfide, metal cyanides, metal
thiocyanates, manganese dioxide,
phosphorus. Violent reaction with organic
matter. When heated to decomposition it
emits very toxic fumes of I and K2O. See
also IODATES. | | Overdosage | Overdose of potassium iodate, an iodized salt used for iodine supplementation in areas endemic for goiter, has been shown to cause profound visual loss and extensive retinal pigmentary abnormalities.78 FA reveals RPE window defects and ERG and VEP testing show marked impairment of retinal function. Visual acuity may improve slowly over several months. | | Purification Methods | It has been crystallised twice from distilled water (3mL/g) between 100o and 0o, dried for 2hours at 140o and cooled in a desiccator. Analytical reagent grade material dried in this way is suitable for use as an analytical standard. |
| | Potassium iodate Preparation Products And Raw materials |
|