- Ruxolitinib Phosphate
-

- $0.00 / 1kg
-
2024-04-29
- CAS:1092939-17-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000kg
- Ruxolitinib phosphate
-

- $8.00 / 1KG
-
2024-04-09
- CAS:1092939-17-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Ruxolitinib Phosphate
-

- $0.00/ kg
-
2023-12-20
- CAS:1092939-17-7
- Min. Order: 1kg
- Purity: 99%, Single impurity<0.1
- Supply Ability: 1 ton
Related articles - What is Ruxolitinib phosphate?
- Ruxolitinib is an oral inhibitor of JAK1 and JAK2, which is approved for the treatment of myeloproliferative neoplasm-associat....
- Feb 10,2020
|
| Product Name: | Ruxolitinib phosphate | | Synonyms: | (betaR)-beta-Cyclopentyl-4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazole-1-propanenitrile phosphate;INCB 018424;Ruxolitinib, Phosphate Salt;Ruxolitinib, Phosphate;(βR)-β-Cyclopentyl-4-(7H-pyrrolo[2,3-d]pyriMidin-4-yl)-1H-pyrazole-1-propanenitrile Phosphate;(3R)-3-cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]propanenitrile phosphoric acid;Ruxolitinib (INCB-18424) phosphate;Jakafi (ruxolitinib) | | CAS: | 1092939-17-7 | | MF: | C17H21N6O4P | | MW: | 404.37 | | EINECS: | | | Product Categories: | Intracellular signaling.;Inhibitors;Antineoplastic;API | | Mol File: | 1092939-17-7.mol | 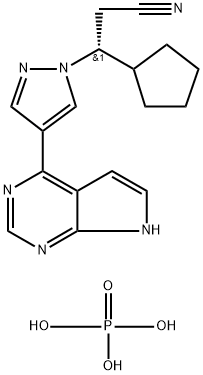 |
| | Ruxolitinib phosphate Chemical Properties |
| Melting point | 186-190°C | | storage temp. | Refrigerator | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | color | White to Off-White |
| | Ruxolitinib phosphate Usage And Synthesis |
| Physical and Chemical Properties | Ruxolitinib, (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3- cyclopentylpropanenitrile phosphate, has a molecular weight of 404.36 kDa. Ruxolitinib is soluble in aqueous solutions at pH 1-8. Ruxolitinib tablets are stable at 20-25°C and tolerate brief exposures to temperatures outside this range, if they stay within 15-30°C.
| | Approval for Use | Ruxolitinib is an oral inhibitor of JAK1 and JAK2, which is approved for the treatment of myeloproliferative neoplasm-associated myelofibrosis, further myeloproliferative neoplasms, polycythemia vera and refractory cancer. In the USA, Canada and EU, ruxolitinib is approved for the treatment of patients with intermediate or high-risk PMF, post-PV MF or post-ET MF. In addition, ruxolitinib recently has been approved for the treatment of patients with PV who have had an inadequate response to or are intolerant of hydroxyurea, based on the results of Phase II and III clinical studies. To date, ruxolitinib has been approved for indications in MF in 83 countries.
| | Side Effects | Common side effects of ruxolitinib treatment include anaemia and thrombocytopenia. The decline of circulating erythrocytes following ruxolitinib treatment could result in part from stimulation of eryptosis, the suicidal erythrocyte death characterized by cell shrinkage and cell membrane scrambling with phosphatidylserine translocation to the cell surface. Cellular mechanisms involved in the execution of eryptosis include oxidative stress, Ca2+ entry with increase of cytosolic Ca2+ activity ([Ca2+]i), ceramide, decline of cytosolic ATP, caspases, stimulated activity of casein kinase 1α, Janus-activated kinase JAK3, protein kinase C, and p38 kinase, as well as impaired activity of AMP activated kinase AMPK, cGMP-dependent protein kinase, PAK2 kinase and sorafenib/sunitinib sensitive kinases.
| | Uses | The phosphate salt of Ruxolitinib (R702000). Ruxolitinib is a selective Janus tyrosine kinase (JAK1 and JAK2) inhibitor used in the treatment of myeloproliferative neoplasms and psoriasis. | | Definition | ChEBI: A phosphate salt obtained by reaction ruxolitinib with one equivalent of phosphoric acid. Used for the treatment of patients with intermediate or high-risk myelofibrosis, including primary myelofibrosis, post-polycythemia vera myelofibrosis and post-essent
al thrombocythemia myelofibrosis. | | Clinical Use | Ruxolitinib phosphate is a potent, selective, ATP competitive
inhibitor of tyrosine-protein kinases JAK1 and JAK2 which acts by
attenuating cytokine signaling and promotes apoptosis. Ruxolitinib
was discovered and developed by Incyte, is marketed under the
brand name Jakafi,�and is approved for the treatment of patients
with myelofibrosis (MF), including primary MF, post-polycythemia
vera MP, and post-essential thrombocythemia MF. Ruxolitinib
is also undergoing clinical evaluation against a wide variety
of cancer indications including metastatic prostate cancer, pancreatic
cancer, multiple myeloma, leukemia, non-Hodgkin lymphoma, and breast cancer. Additionally, ruxolitinib is being evaluated for
the treatment of psoriasis and thrombocytopenia. | | Synthesis | Ruxolitinib contains
one chiral center, and three general strategies for its preparation
have been reported.165¨C167 These include a racemic synthesis
followed by chiral separation or resolution, introduction of the side
chain via an aza-Michael addition of the pyrazole fragment to 3-
cyclopentylpropiolonitrile and asymmetric hydrogenation of the
resulting alkene, and through introduction of the side chain via
an organocatalytic, asymmetric aza-Michael addition. The route
described herein utilizes the first strategy as this appears
to be the largest scale reported.
The synthesis was initiated by SEM protection of commercially
available chloropyrrolopyrimidine 201 to provide the protected
chloropyrrolopyrimidine 202 in 89% yield. Suzuki coupling of 202
with the pyrazole pinacolatoboronate 203 gave pyrazole 204 in
64% yield. aza-Michael reaction of pyrazole 204 with 3-cyclopentylacrylonitrile
205 was accomplished in the presence of DBU to furnish
SEM-protected ruxolitinib 206 in 98% yield as the racemate.
The desired enantiomer 207 was isolated via chiral column separation
in 93.5% yield and 99.4% ee, on 100 kg scale. Removal of the
SEM group was accomplished through a two step process via treatment
with lithium tetrafluoroborate and aqueous ammonium
hydroxide, ultimately giving rise to ruxolitinib 208 in 84% yield.
The phosphate salt was then prepared by treatment with phosphoric
acid. Crystallization from MeOH/i-PrOH/n-heptane gave ruxolitinib
phosphate (XX) in good overall yield in 99.8% ee. Pyrazole
pinacolatoboronate 203 was prepared from pyrazole 209 via iodination
with N-iodosuccinimide followed by reaction with trimethyl
silyl chloride to give protected iodopyrazaole 210 in high yield.
Reaction of 210 with i-PrMgCl to form the corresponding Grignard
reagent followed by reaction with isopropylpinacolborane 211
provided Suzuki boronate synthon 203 in 55% yield.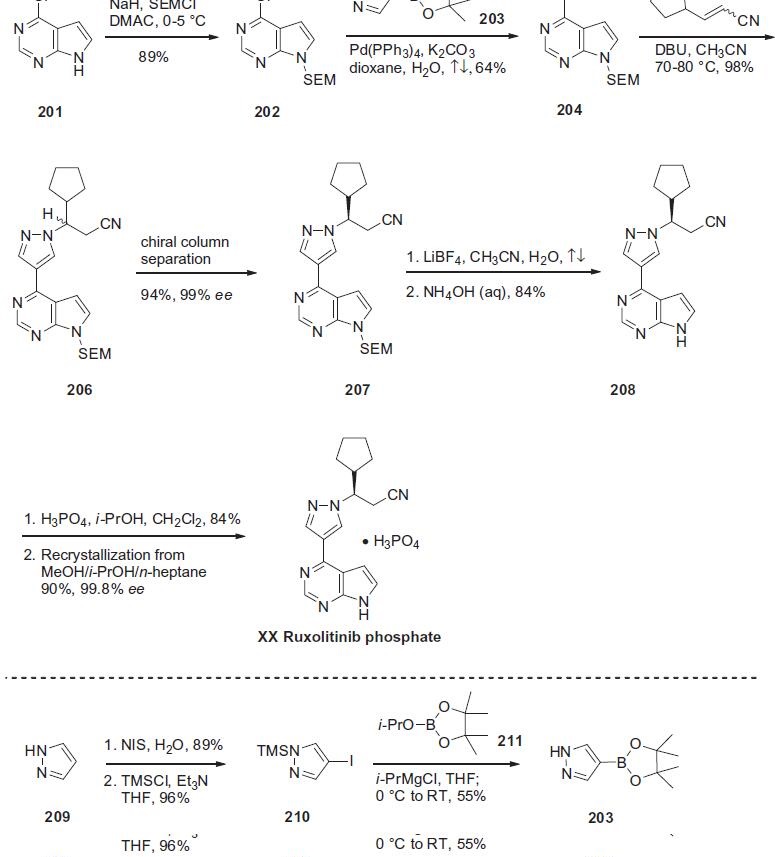
| | storage | Store at -20°C |
| | Ruxolitinib phosphate Preparation Products And Raw materials |
|