- Pimavanserin
-

- $650.00 / 1Kg/Bag
-
2024-04-22
- CAS:706779-91-1
- Min. Order: 1Kg/Bag
- Purity: 99% up, High Density
- Supply Ability: 20 tons
- Pimavanserin
-
- $35.00 / 1kg
-
2024-03-23
- CAS:706779-91-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Pimavanserin
-

- $0.00 / 1g
-
2024-03-12
- CAS:706779-91-1
- Min. Order: 1g
- Purity: 98% HPLC
- Supply Ability: 100kg
|
| | Pimavanserin Basic information |
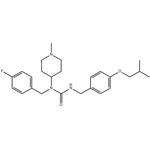
| Product Name: | Pimavanserin | | Synonyms: | Pimavanserin;PIMAVANSERIN/1-(4-FLUOROBENZYL)-3-(4-ISOBUTOXYBENZYL)-1-(1-METHYLPIPERIDIN-4-YL)UREA;Unii-jz963p0dik;Pimavamserin;1-[(4-fluorophenyl)Methyl]-1-(1-Methylpiperidin-4-yl)-3-{[4-(2-Methylpropoxy)phenyl]Methyl}urea;N-(4-Fluorobenzyl)-N-(1-Methylpiperidin-4-yl)-N'-[[4-(2-Methylpropyloxy)phenyl]Methyl]carbaMide;N-[(4-Fluorophenyl)Methyl]-N-(1-Methyl-4-piperidinyl)-N'-[[4-(2-Methylpropoxy)phenyl]Methyl]urea;Urea,N-[(4-fluorophenyl)Methyl]-N-(1-Methyl-4-piperidinyl)-N'-[[4-(2-Methylpropoxy)phenyl]Methyl]- | | CAS: | 706779-91-1 | | MF: | C25H34FN3O2 | | MW: | 427.55 | | EINECS: | 1806241-263-5 | | Product Categories: | Intermediates & Fine Chemicals;Pharmaceuticals;Inhibitors | | Mol File: | 706779-91-1.mol |  |
| | Pimavanserin Chemical Properties |
| Melting point | 100-103oC | | Boiling point | 604.2±55.0 °C(Predicted) | | density | 1.15±0.1 g/cm3(Predicted) | | storage temp. | Refrigerator | | solubility | Methanol (Slightly) | | pka | 13.52±0.46(Predicted) | | form | Solid | | color | Pale Yellow to Pale Orange | | InChI | InChI=1S/C25H34FN3O2/c1-19(2)18-31-24-10-6-20(7-11-24)16-27-25(30)29(23-12-14-28(3)15-13-23)17-21-4-8-22(26)9-5-21/h4-11,19,23H,12-18H2,1-3H3,(H,27,30) | | InChIKey | RKEWSXXUOLRFBX-UHFFFAOYSA-N | | SMILES | N(CC1=CC=C(F)C=C1)(C1CCN(C)CC1)C(NCC1=CC=C(OCC(C)C)C=C1)=O | | CAS DataBase Reference | 706779-91-1(CAS DataBase Reference) |
| Hazard Codes | Xi | | Safety Statements | 24/25 | | HS Code | 29339900 |
| | Pimavanserin Usage And Synthesis |
| Description | Pimavanserin is a kind of novel oral administrated drug for treatment of Parkinson's psychosis developed by Acadia pharmaceutical Company (US) with the trade name being Pimavanserin. In September 3rd 2014, it has been granted by the US Food and Drug Administration (FDA) for breakthrough therapy certification. Breakthrough therapy certification is created by the FDA for accelerating the development and review of new drugs for treatment of serious or life-threatening diseases.
Currently there are about seven millions to ten million of patients of Parkinson's disease worldwide with China contributing 2.6 million, ranking first in the world with 100,000 new patients emerging every year. More than 50% of patients of Parkinson's disease have had psychiatric symptoms (PDP). These psychiatric symptoms are mainly manifested as hallucinations and delusions, bringing greater challenge to the treatment and care for patients of Parkinson's disease. Dopamine is the primary target for the treatment of Parkinson's disease because most antipsychotics drugs will block the dopamine in the brain of the patients of Parkinson’s disease, making their situation of motor dysfunction be even worse, and thus currently not being suitable for these patients.
 | | Chemical Properties | Yellow Solid | | Uses | Drug used in the treatment of Parkinson’s disease and psychosis. | | Definition | ChEBI: Pimavanserin is a member of the class of ureas in which three of the four hydrogens are replaced by 4-fluorobenzyl, 1-methylpiperidin-4-yl, and 4-(isopropyloxy)benzyl groups. An atypical antipsychotic that is used (in the form of its tartrate salt) for treatment of hallucinations and delusions associated with Parkinson's disease. It has a role as an antipsychotic agent, a 5-hydroxytryptamine 2A receptor inverse agonist and a serotonergic antagonist. It is a member of ureas, a member of piperidines, a member of monofluorobenzenes, an aromatic ether and a tertiary amino compound. It is a conjugate base of a pimavanserin(1+). | | General Description | Pimavanserin(706779-91-1) is a novel first-line drug of antipsychotic symptoms belongs to non-dopaminergic neurotransmitters with the drug targets being serotonin 2A receptor (5-HT2A), which can selectively block the 5-HT 2A receptor without affecting the action of dopamine with the routine administration being treatment via oral administration once daily. There are evidences showing that the drug is quite effective in the treatment of Parkinson's disease psychosis with well tolerance. Moreover, it does not block the dopamine receptors, and therefore does not lead to worsening of symptoms of Parkinson's disease. A 6-week-lasting randomized, double-blind, placebo-controlled trial has included 199 patients and evaluated the safety and efficacy of pimavanserin. | | Biological Activity | Pimavanserin is an inverse agonist of the serotonin (5-HT) receptor subtype 5-HT2A (IC50 = 1.86 nM; Ki = 0.5 nM). It is selective for 5-HT2A over a panel of 65 ion channels, enzymes, and receptors (Kis = >100 nM). Pimavanserin reduces head-twitch behavior and prepulse inhibition deficits induced by the 5-HT2A receptor agonist DOI in rats at doses of 3 mg/kg, p.o. and 1-10 mg/kg, s.c., respectively. It also exhibits antipsychotic-like activity, reducing hyperactivity induced by (+)-MK-801 in mice. Pimavanserin acts synergistically with haloperidol or risperidone to suppress (+)-MK-801-induced hyperactivity and attenuates haloperidol- and risperidone-induced catalepsy in rats. Formulations containing pimavanserin have been used for the treatment of psychosis in Parkinson''s disease. | | Side effects | Pimavanserin is a medication used to treat hallucinations and delusions associated with Parkinson's disease psychosis. Like all medications, it carries the risk of side effects. Common side effects of pimavanserin may include:
Bloating or swelling of the face, arms, hands, lower legs, or feet
confusion
nausea
rapid weight gain
unusual weight gain or loss
www.mayoclinic.org | | Enzyme inhibitor | This orally active atypical non-dopaminergic antipsychotic[ (FWfree-base = 427.56 g/mol; CAS 706779-91-1 (free base) and 706782-28-7 (tartrate salt)), also known as ACP-103, Nuplazid? and N-(4-fluorophenylmethyl)- N-(1-methyl-piperidin-4-yl)-N'-(4-(2-methylpropyloxy)phenyl-methyl)carbamide, is a potent inverse agonist and antagonist at serotonin 5-HT2A receptors (Ki = 0.087 nM) and less so at serotonin 5-HT2C receptors (Ki = 0.44 nM). Pimavanserin shows low binding to σ1 receptors (Ki = 120 nM), with no appreciable affinity (Ki > 300 nM) to serotonin 5-HT2B, dopaminergic (including D2), muscarinic, histaminergic, or adrenergic receptors, or to calcium channels. 5-HT2A receptor dysregulation is implicated in both the etiology and treatment of schizophrenia, and the same receptor plays an active role in the regulation of sleep architecture. Nuplazid is specifically indicated in its FDA approval (April, 2016) for the treatment of hallucinations and delusions associated with Parkinson disease. Significantly, 5-HT2A serotonin receptor antagonists differentially regulate 5-HT2A receptor protein level in vivo. See also Ketanserin; RH-34; Risperidone; Ritanserin; Setoperone; Volinanserin. | | storage | Store at -20°C | | Mode of action | The mechanism of action of pimavanserin in the treatment of hallucinations and delusions associated with Parkinson’s disease psychosis is unknown. However, the effect of pimavanserin could be mediated through a combination of inverse agonist and antagonist activity at serotonin 5-HT2A receptors and to a lesser extent at serotonin 5-HT2C receptors.
www.accessdata.fda.gov |
| | Pimavanserin Preparation Products And Raw materials |
|