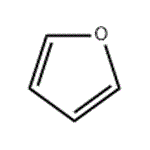
- Furan
-
- $1.10 / 1g
-
2023-07-27
- CAS:110-00-9
- Min. Order: 1g
- Purity: 99.0% Min
- Supply Ability: 100 Tons
- Furan
-

- $200.00 / 1kg
-
2023-06-26
- CAS:110-00-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg/Month
- Furan
-

- $200.00 / 1kg
-
2023-05-27
- CAS:110-00-9
- Min. Order: 1kg
- Purity: 99.99%
- Supply Ability: 50000 tons
Related articles - Electrophilic Reactions of Furan
- Furans are fairly soluble in organic solvents, including alcohol and ether, but slightly soluble in water. They are toxic and ....
- Jan 24,2022
- Uses of Furan
- Furan is a five-membered aromatic oxygen heterocycle, comprised of four conjugated carbon atoms and one oxygen atom in a cycli....
- Jan 24,2022
- Toxicity and hazards of Furan
- Furan can cause eye, skin, and mucus membrane irritation, burning sensation and, in severe cases, corrosion. If inhaled, furan....
- Oct 27,2021
Question and answer - Q:Furan in Foods
- A:Furan is a colorless, volatile liquid used in some chemical manufacturing industries. Furan has occasionally been reported to ....
- Sep 20,2019
|
| | Furan Chemical Properties |
| Melting point | -85.6 °C | | Boiling point | 67 °C10 mm Hg(lit.) | | density | 0.936 g/mL at 25 °C(lit.) | | vapor density | 2.35 (vs air) | | vapor pressure | 1672 mm Hg ( 55 °C) | | refractive index | n20/D 1.5070(lit.) | | Fp | 160 °F | | storage temp. | Store below +30°C. | | solubility | alcohols: freely soluble | | form | Liquid | | color | Clear colorless to yellow | | Specific Gravity | 0.94 | | Odor | Peculiar spicy -smokey, slightly Cinnamon-like odor | | Odor Threshold | 9.9ppm | | explosive limit | 2.3-14.3%(V) | | Water Solubility | insoluble | | Sensitive | Air & Light Sensitive | | Merck | 14,4296 | | BRN | 103221 | | Dielectric constant | 3.0(25℃) | | Exposure limits | NIOSH: IDLH 13 ppm | | Stability: | Stable. Substances to be avoided include strong oxidising agents, acids, peroxides and oxygen. Highly flammable; can form explosive mixtures with air. | | InChIKey | YLQBMQCUIZJEEH-UHFFFAOYSA-N | | LogP | 1.34 | | CAS DataBase Reference | 110-00-9(CAS DataBase Reference) | | IARC | 2B (Vol. 63) 1995 | | NIST Chemistry Reference | Furan(110-00-9) | | EPA Substance Registry System | Furan (110-00-9) |
| | Furan Usage And Synthesis |
| Chemical Properties | Furan is the simplest five membered oxygen-containing heterocyclic compounds with its molecular structure containing ring diene ether. It belongs to 6π electron-based compound with all the 5 atoms being sp2-hybrid and located in the same plane. The pair of non-shared electrons of the oxygen atoms occupied p-orbital which is perpendicular to the ring plane and is parallel and overlapping with the p-orbital of the four carbons atoms, forming a five-atoms& six-electrons conjugated closed system. Therefore, it has a certain degree of aromatic nature. However, due to the electron density on the ring is not as uniform as benzene; it has a more active chemical property than benzene and can participate in many kinds of chemical reactions. It should be especially noted that, its 2, 5-position contains high electron density and prone to have nucleophilic substitution reaction. Moreover, all the derivatives of almost happens in 2-position such as furfural, furfuryl alcohol and furan carboxylic acid. Furan can also have nitrification, halogenated, sulfonated reaction. Its diene bonds can also participate in addition reaction, for example, have reaction with maleic anhydride, having diene addition reaction and generating 7-bicyclo-[2.2.1]-hept-5-ene-2, 3-dicarboxylic anhydride.
Under acidic condition, furan can have hydrolysis and ring open, producing 1, 4-dicarbonyl compounds. But once the furan ring has been introduced of electrophilic group, its activity can be greatly reduced and will not have diene reaction even encountering strong pro-diene compound. Although it contains certain aromatic property, but this property is very weak and there are no free hydroxyl furan or amino furan like phenol and aniline. When furan is subject to hydrogenation, it can give rise to two kinds of hydrides, namely dihydrofuran (2, 3-dihydrofuran and 2, 5-dihydrofuran) and tetrahydrofuran with the former being partially saturated cyclic enol ether and the later being saturated. Furan has a high thermal stability and is decomposed only at a temperature as high as 670 ℃ in the absence of catalyst. In the presence of a nickel catalyst, it is decomposed when heated to 360 ℃. This product is toxic and carcinogenic. The lethal concentration for Rat through oral administration is 300mg/kg. Its steam is narcotic which is similar as ether. It is easy to generate explosive peroxides in air.
Its steam encounters pine wood chip pre-soaked with hydrochloride tablets and generate green color (pine wood chip reaction). This can be used to identify the presence of furan.
The above information is edited by the chemicalbook of Dai Xiongfeng.
| | Raw material for organic synthesis | Furan is an important raw material in organic synthesis industry and can be used in the manufacture of many kinds of industrial chemical product such as pyrrole, thiophene tetrahydrofuran and benzofuran. It can also be applied to the manufacture of drugs such as anisodamine, atropine, toluoyl pyrrole sodium acetate, thiamine furan and furadantin and so on. Furan, through oxidation and polymerization, can generate conductive poly-furan polymer with the conductivity being 18S/m (use AsF5 as the oxidant) or 2.2 × 10-4S/m (with NOSbF6 as the oxidant). Furan is unstable and prone to be subject to oxidation and polymerization. Furan, via catalytic hydrogenation, can generate tetrahydrofuran.
Many furan derivatives are drugs such as furfural, furfuryl alcohol, furan carboxylic acid, nitrofurazone, furapromide and furosemide and so on. In some monosaccharides such as fructose and ribose as well as their derivatives, there exists the cyclic structure of hydrogenated furans.
| | Uses | It is primarily used for the adhesive for the production of large quantities of hot-box technological cores.
Furan can be used as the raw material of organic synthesis and solvents. Furan can also be used for preparation of pyrrole, thiophene and tetrahydrofuran. Tetrahydrofuran is an important derivative of furan with many important applications. Furan, when undergoes etherfication and reduction to give 2, 5-dimethoxy dihydrofuran which is further converted to 2-hydroxy-1, 4-butanedial through hydrolysis, can be used for the production of anisodamine via synthetic approach; When furan undergoes etherfication and reduction to give 2, 5-dimethoxy tetrahydrofuran and generate butanedial via hydrolysis, is the raw material for synthesis of another kind of alkaloid, atropine. Furan is also used to produce anti-inflammatory drugs toluoyl pyrrole sodium acetate with production of each ton of this product consuming 4.75 t of furan.
| | Production method | Furfural is oxidized into 2-furan formic acid which undergoes decarboxylation to obtain furans. Heat the 2-furan formic acid to 200-205 ℃ (around the boiling point) so it is decomposed into carbon dioxide and furans. During the reaction process, send the sublimated 2-furan formic acid back to the reactor with the distilled furan undergoing re-distillation; collect the distillation fraction of 31-34 ℃ which is the relative pure finished product with the overall yield being about 75%. Industrially, it can be obtained through direct decarbonylation of furfural. The catalyst used in the reaction includes aluminum silicate, metal oxides or hydroxides as well as the mixtures of alloy or metals such as the mixture of zinc chromite and manganese. The reaction temperature should be about 400 ℃ with the yield being 90%. For large-scale production, the yield can be 74%.
| | Category | Flammable liquid
| | Toxicity grading | poisoning
| | Acute toxicity | Inhalation-rat LC50: 3398 PPM/per hour; Inhalation-Mouse LC50: 120 mg/m3/per hour.
| | Explosive and Hazardous characteristics | Mixing with air can be explosive.
| | Flammability and hazard characteristics | It is flammable in case of fire, high temperature and oxidant with burning releasing irritated fume.
| | Storage characteristics | Treasury ventilation low-temperature and dry; store it separately from oxidants and acids.
| | Extinguishing agent | Dry powder, dry sand, carbon dioxide, foam, 1211 fire extinguishing agent
| | Professional Standards | TLV-TWA 0.5 mg/m3 | | Description | Furan occurs naturally in oils distilled from rosin containing
pinewood. In addition, many natural foods contain the furan
ring structure and substituted furans may be formed through
cooking of simple carbohydrates. Furan is also found in
tobacco smoke as well as wood smoke and gas emissions from
gasoline and diesel engines. Furan has also been detected in
industrial effluents and can be emitted to the air from petroleum
refineries and coal-mining and gasification plants. | | Chemical Properties | Furan is a colorless liquid; turns brown on
standing. Strong ethereal odor. | | Chemical Properties | Furan is a cyclic dienic ether stabilized by benzene-like resonance. Because of its conjugated unsaturation and heterocyclic atom, furan will undergo many types of reactions. It is, therefore, of interest as a chemical intermediate for pharmaceuticals, insecticides and fine chemicals. The heterocyclic oxygen atom in a ring with conjugated unsaturation gives furan a combination of ether, aromatic and olefinic characteristics. This polyfunctionality permits it to undergo a variety of reactions. Compared to benzene, the furan ring has greater reactivity, and is more susceptible to cleavage, thus resembling the vinyl ethers. Like the vinyl ethers, the furan ring is cleaved by aqueous acids. This reaction is accompanied by resinification . | | Uses | Furan is a five-membered heterocyclic aromatic ring. Furan is used as a building block for the preparation of many heterocyclic compounds. | | Uses | Furan is a valuable chemical intermediate for pharmaceuticals, insecticides, and
other organic compounds. The furan ring is cleaved by aqueous acid which can lead
to polymer formation. | | Uses | Furan is used as an intermediate in organicsynthesis. | | Uses | In organic syntheses. Furan is occasionally used in the composing of artificial essential oils. It finds a
little - rare - use in industrial fragrances for
certain masking purposes, e. g. when the
problem is: to mask very volatile, undesirable
odors. It is used in special
fragrances such as “smokey” odors for certain
products, directly or indirectly connected with
food products. | | Preparation | By decarboxylation of Furoic acid. Furan can also be prepared directly from
Furfural. | | Definition | furan: A colourless liquid compound,C4H4O; r.d. 0.94; m.p. –86°C;b.p. 31.4°C. It has a five-memberedring consisting of four CH2 groupsand one oxygen atom. | | Definition | A heterocyclic liquid organic
compound. Its five-membered ring contains
four carbon atoms and one oxygen
atom. The structure is characteristic of
some monosaccharide sugars (furanoses). | | Definition | A heterocyclic compound. | | General Description | Furan is a stable, colourless chemical liquid. It is highly flammable and forms explosive mixtures with air. It is incompatible with many other chemical substances, for example, strong oxidising agents, acids, peroxides, and oxygen. Furan is produced commercially by decarbonylation of furfural. It is used mainly in the production of tetrahydrofuran, thiophene, and pyrrole. It also occurs naturally in certain woods and during the combustion of coal and is found in engine exhausts, wood smoke, and tobacco smoke. | | Air & Water Reactions | Highly flammable. When uninhibited, Furan forms explosive peroxides on exposure to air. Insoluble in water. | | Reactivity Profile | Furan is sensitive to heat and may turn brown upon standing. Furan may be light sensitive. When uninhibited, Furan forms explosive peroxides on exposure to air. Furan may react with oxidizers, acids, peroxides and oxygen. Furan resinifies on evaporation or when in contact with mineral acids, but Furan is stable in alkalis. . | | Hazard | Flammable, dangerous fire risk, flammable
limits 2–24%, forms peroxides on exposure to air.
Absorbed by skin. Possible carcinogen. | | Health Hazard | Furan is a highly toxic compound. Inhalationof its vapors can cause acute pulmonaryedema and lung damage. The intraperitonealLD50 value in rodents is between 5 and7 mg/kg. The inhalation LC50 value in micefor a 1-hour exposure is 120 mg/m3. | | Health Hazard | The vapors are narcotic. Acute exposure to Furan by inhalation may involve both reversible and irreversible changes. Acute exposure by ingestion or skin absorption, as well as chronic exposure, are associated with high toxicity. | | Fire Hazard | Very dangerous, upon exposure to heat or flame. Furan may form unstable peroxides on exposure to air. Contact with acids can initiate a violent, heat producing reaction. Avoid acids, oxidizing agents. Upon standing in air, Furan may form unstable peroxides. | | Flammability and Explosibility | Extremely flammable | | Safety Profile | Confirmed carcinogen.
Poison by inhalation and intraperitoneal
routes. Moderately toxic by ingestion and
skin contact. Experimental reproductive
effects. A narcotic. Mutation data reported.
The exposure concentration limit of 10 ppm
together with its low boiling point requires
that adequate ventilation be provided in
areas where ths chemical is handled.
Contact with liquid must be avoided since
this chemical can be absorbed through the
skin. Washing thoroughly with soap and
water followed by prolonged rinsing should
be done immedlatelp after accidental
contact.
A very dangerous fire hazard when
exposed to heat or flame; can react with
oxidzing materials. Unstabdized, it may
form unstable peroxides on exposure to air and should always be tested before
ddlation. Washing with an aqueous
solution of ferrous sulfate slightly acidified
with sodum bisulfate will remove these
peroxides. Confirm by test. Contact with
acids can initiate a violent exothermic
reaction. Moderate explosion hazard when
exposed to flame. Furan's low bohg point
makes it easy to obtain explosive
concentrations of the vapor in inadequately
ventilated areas. To fight fire, use CO2, dr).
chemical. When heated to decomposition it
emits acrid smoke and irritating fumes. See
also PEROXIDES. | | Potential Exposure | Furan is used as a chemical intermedi ate in the production of herbicides and pharmaceuticals; for
making tetrahydrofuran; in formation of lacquers; as a sol vent for resins in organic synthesis, especially for pyrrole,
thiophene. | | First aid | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray. | | Carcinogenicity | Furan is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals. | | Environmental Fate | Furan may be released to the environment as a waste industrial
product or from unintentional or accidental releases. If released
to soil, it is expected to volatilize. If released to water, furan is not
expected to adsorb to suspended particles and sediment and is
likely to volatilize to ambient air. Sulfate-reducing bacteria can
degrade furan. However, under nonsulfate-reducing conditions,
biodegradation in soil and water is expected to be slow. In the
air, furan will exist as a vapor and will be subject to degradation
by reacting with hydroxyl radicals. | | storage | Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with this chemical you should be trainedon its proper handling and storage. Before entering confinedspace where this chemical may be present, check to makesure that an explosive concentration does not exist. Store inan explosion-proof refrigerator. Keep in a tightly closedcontainer under an inert atmosphere and protect from lightfor long-term storage. A regulated, marked area should beestablished where this chemical is handled, used, or storedin compliance with OSHA Standard 1910.1045. | | Shipping | UN2389 Furan, Hazard Class: 3; Labels:
3-Flammable liquid. | | Purification Methods | Shake it with aqueous 5% KOH, dry it with CaSO4 or Na2SO4, then distil it under nitrogen, from KOH or sodium, immediately before use. A trace of hydroquinone could be added as an inhibitor of oxidation. [Beilstein 17 H 27, 17 I 16, 17 II 34, 17/1 V 291.] | | Toxicity evaluation | Furan can cause eye, skin, and mucus membrane irritation,
burning sensation and, in severe cases, corrosion. If inhaled,
furan may produce pulmonary edema and bronchiolar
necrosis. When absorbed, furan can cause central nervous
system (CNS) depression to the point of narcosis and tonic
seizures.
Furan is metabolized by the cytochrome P450 enzymes
in the liver and other tissues. Furan is metabolized to cis-2-
butene-1,4-dial. This active metabolite is acutely toxic to
liver cells. The furan ring undergoes oxidative cleavage and
forms highly reactive furan radical cations or epoxides,
which react directly with cellular nucleophiles. These reactive
metabolites may react directly with deoxyribonucleic acid
(DNA) or with cellular proteins to produce disruption of
cellular functions and cell death. Chronic cell death and
regeneration produced by chronic furan exposure may be
a significant factor in the carcinogenicity potential of the
chemical. In addition, there is some evidence to suggest that
the reactive metabolites of furan may induce mutations in
cellular genes. | | Incompatibilities | May form explosive mixture with air.
Incompatible with oxidizers (chlorates, nitrates, peroxides,
permanganates, perchlorates, chlorine, bromine, fluorine,
etc.); contact may cause fires or explosions. Keep away
from alkaline materials, strong acids, amines, strong bases,
reducing agents. Unless stabilized with an inhibitor, air
exposure forms unstable peroxides. | | Waste Disposal | Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform with EPA regulations govern ing storage, transportation, treatment, and waste disposal. |
| | Furan Preparation Products And Raw materials |
| Raw materials | Furfural-->2-Furoic acid-->Metallic oxides-->Zinc chromate-->CHROMIUM(II) CHLORIDE | | Preparation Products | 2,5-Dimethoxytetrahydrofuran-->Carbofuran-->Pyrrole-->2-[3-(2-FURYL)PHENYL]-4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLANE-->FENOTHIOCARB-->Fluvastatin sodium salt-->polypyrrole-polyvinyl chloride composite film-->Cefuroxime-->Suprofen-->2-Bromofuran-->2-Acetylfuran-->2-NITROFURAN-->Efavirenz-->1,4-EPOXY-1,4-DIHYDRONAPHTHALENE-->3-CHLORO-2-METHYLBIPHENYL-->Cladribine-->2-PROPIONYLFURAN-->Cefetamet hydrochloride-->Gabexate mesylate-->Bispyribac-sodium-->2-HEXANOYLFURAN-->Cabergoline-->EXO-3,6-EPOXY-1,2,3,6-TETRAHYDROPHTHALIC ANHYDRIDE-->DELAVIRDINE |
|