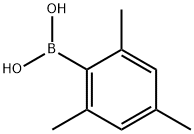
2,4,6-Trimethylphenylboronic acid synthesis
- Product Name:2,4,6-Trimethylphenylboronic acid
- CAS Number:5980-97-2
- Molecular formula:C9H13BO2
- Molecular Weight:164.01

121-43-7
342 suppliers
$14.00/25mL
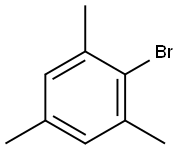
576-83-0
251 suppliers
$27.00/25G

5980-97-2
258 suppliers
$10.00/1g
Yield:5980-97-2 85%
Reaction Conditions:
Stage #1: 2,4,6-trimethylphenyl bromidewith magnesium in tetrahydrofuran; for 3 h;Reflux;
Stage #2: Trimethyl borate in tetrahydrofuran at -78 - 20; for 12 h;
Steps:
General procedure of boronic acid synthesis
General procedure: A solution of 1 equiv. (10 mmol) mesitylbromide and 5 ml THF was addeddropwise to 1.2 equiv. (12 mmol, 0.30g) activated Mg turnings in 10 ml THF.After the reaction started, the mixture was refluxed for 3 hours. Then the reactionmixture was cooled to -78°C, and treated with 2 equiv. (20 mmol, 2.0g) offreshly distilled trimethylborate, and then the reaction mixture was allowed to warm up toroom temperature, and stirred overnight (12 hours). The reaction was quenched with 2 equiv.(20 mmol, 20 ml) 1M HCl-solution at 0 °C, then the mixture was stirred at rt. for two hours.The phases were separated and the aqueous phase was washed with 3x10 ml diethyl ether.The combined organic layer was washed with brine three times, dried over anhydrousNa2SO4, filtered off and evaporated to dryness. The resulted solid/oil was suspended in hexane,filtered off, washed with hexane 3 times and dried.
References:
Dorkó, éva;Kótai, Bianka;F?ldes, Tamás;Gy?m?re, ádám;Pápai, Imre;Soós, Tibor [Journal of Organometallic Chemistry,2017,vol. 847,p. 258 - 262] Location in patent:supporting information

576-83-0
251 suppliers
$27.00/25G

5980-97-2
258 suppliers
$10.00/1g

67-56-1
726 suppliers
$9.00/25ml
55124-35-1
23 suppliers
$60.00/100mg

576-83-0
251 suppliers
$27.00/25G

5980-97-2
258 suppliers
$10.00/1g

121-43-7
342 suppliers
$14.00/25mL

7732-18-5
446 suppliers
$13.50/100ML
2633-66-1
46 suppliers
$91.00/100ml

5980-97-2
258 suppliers
$10.00/1g
34907-53-4
22 suppliers
inquiry

5980-97-2
258 suppliers
$10.00/1g