
ANISYL FORMATE synthesis
- Product Name:ANISYL FORMATE
- CAS Number:122-91-8
- Molecular formula:C9H10O3
- Molecular Weight:166.17
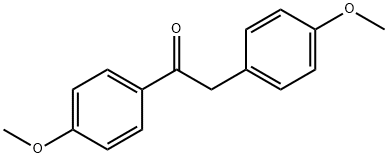
120-44-5
124 suppliers
$10.00/1g

64-17-5
696 suppliers
$10.00/50g
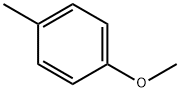
104-93-8
382 suppliers
$6.00/25g
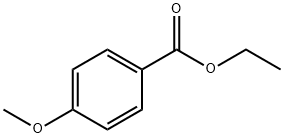
94-30-4
172 suppliers
$20.00/25g

122-91-8
76 suppliers
$50.00/1 SAMPLE-K
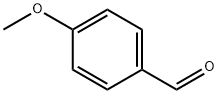
123-11-5
828 suppliers
$10.00/10g
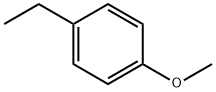
1515-95-3
132 suppliers
$33.00/5g
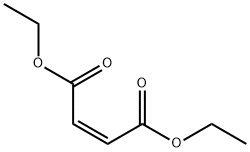
141-05-9
440 suppliers
$6.00/25g
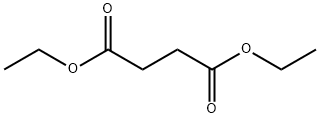
123-25-1
443 suppliers
$5.00/25g
Yield:-
Reaction Conditions:
with [methyl-3-(butyl-4-sulfonate) imidazolium]CuPW12O40;oxygen at 159.84; under 6000.6 Torr; for 5 h;
Steps:
General procedure for catalytic oxidation of lignin and model compound.
General procedure: In a typical process, 0.25 g lignin, 0.9 mmol POM-IL catalyst and 20 mL 100% ethanol werecharged into a 100 mL stainless autoclave (Andorra MED1220, Premex Co. Ltd.). After airpurging with pure oxygen five times and pressurizing to 0.8 MPa, the reactor was heated to thedesignated temperature and maintained for the desired time. Once the latter elapsed, the autoclavewas cooled rapidly to room temperature in an ice water bath. The reaction mixture was removedand the reactor was washed with anhydrous ethanol (3 5.0 mL). The IL catalyst was precipitatedat room temperature and used for the next run after drying (extra fresh catalyst was added tooffset transfer losses). The liquid mixture was then diluted by ethanol to 50 mL for qualitative andquantitative analysis, while dimethyl phthalate was used as the internal standard. When aqueoussolutions of ethanol were used, the spent mixture was rotary evaporated under reduced pressurefor solvent recovery. The concentrated liquor was esterified with 10 mL anhydrous ethanol at 373K for 2 h and then diluted to 50 mL with ethanol. Volatile products were qualitatively andquantitatively analyzed via gas chromatography-mass spectrometry (GC-MS) and gaschromatography-flame ionization detection (GC-FID). Residual lignin can be obtained throughsimple precipitation processes. Organosolv lignin was recovered as follows: 60 mL deionized water was added into 20 mL of the above reaction mixture causing precipitation. The mixture wasthen separated using centrifugation and was dried until a constant weight was obtained. For therecovery of dealkaline lignin the mixture obtained after reaction was acidified to pH=2 with 1.0mol L-1 HCl solution and the same procedure described for organosolv lignin was conducted.In the atmosphere investigation, a mixture of nitrogen and oxygen with various molar ratioswas used, while depolymerization of lignin was conducted at 433 K for 5.0 h in the single stageexperiments. For a typical two-stage process, the lignin was first depolymerized employing theaforementioned conditions. When the mixture was cooled to room temperature, an extra 0.8 MPanitrogen or oxygen was purged into the reactor and the reaction was heated to 433 K for 1.0 or 2.0h. The product separation and analysis procedure remained unchanged to that describedpreviously. In comparative and control experiments, a series of model compounds (monolignolsand potential intermediate products) were tested under the same procedures as that for lignin (i.e.,0.25 g model compound, 0.9 mmol POM-IL catalyst and 20 mL 100% ethanol solvent). Triplicateexperiments were conducted and the data shown in this study is the average.
References:
Cai, Zhenping;Long, Jinxing;Li, Yingwen;Ye, Lin;Yin, Biaolin;France, Liam John;Dong, Juncai;Zheng, Lirong;He, Hongyan;Liu, Sijie;Tsang, Shik Chi Edman;Li, Xuehui [Chem,2019,vol. 5,# 9,p. 2365 - 2377] Location in patent:supporting information

64-18-6
1056 suppliers
$22.12/250ML
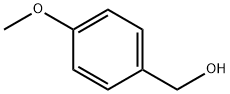
105-13-5
540 suppliers
$5.00/5 g

122-91-8
76 suppliers
$50.00/1 SAMPLE-K

105-13-5
540 suppliers
$5.00/5 g
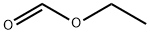
109-94-4
464 suppliers
$10.00/10g

122-91-8
76 suppliers
$50.00/1 SAMPLE-K
![2H-Pyran, tetrahydro-2-[(4-methoxyphenyl)methoxy]-](/CAS/20210305/GIF/18494-82-1.gif)
18494-82-1
1 suppliers
inquiry
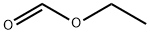
109-94-4
464 suppliers
$10.00/10g

122-91-8
76 suppliers
$50.00/1 SAMPLE-K
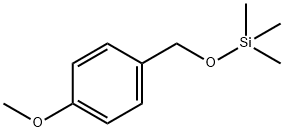
14629-56-2
1 suppliers
inquiry
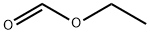
109-94-4
464 suppliers
$10.00/10g

122-91-8
76 suppliers
$50.00/1 SAMPLE-K