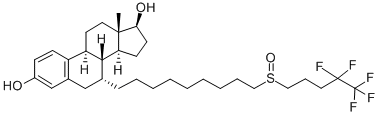
Application and Pharmacology of Fulvestrant
Oct 9,2019
Fulvestrant is a drug treatment of hormone receptor (HR)-positive metastatic breast cancer in post-menopausal women with disease progression following anti-estrogen therapy. It is an estrogen receptor antagonist with no agonist effects, which works both by down-regulating and by degrading the estrogen receptor.
Application
Fulvestrant is used for the treatment of hormone receptor positive metastatic breast cancer or locally advanced unresectable disease in postmenopausal women; it is given by injection.A 2017 Cochrane review found it is as safe and effective as first line or second line endocrine therapy.
It is also used to treat HR-positive, HER2-negative advanced or metastatic breast cancer in combination with palbociclib in women with disease progression after first-line endocrine therapy.Due to the medication's having a chemical structure similar to that of estrogen, it can interact with immunoassays for blood estradiol concentrations and show falsely elevated results.This can improperly lead to discontinuing the treatment.
Pharmacology
Mechanism of Action
Breast cancer: Competitively binds to estrogen receptors on tumors and other tissue targets, producing nuclear complex that decreases DNA synthesis and inhibits estrogen effects; no estrogen-receptor agonist activity; downregulates estrogen receptors and inhibits breast tumor growth
Precocious puberty (off-label): Estrogen receptor antagonist
Absorption
Peak Plasma Time: 7 days
Duration: Plasma levels detected for 1 month
Distribution
Protein Bound: 99%
Vd: 3-5 L/kg
Metabolism
Via multiple hepatic pathways
Excretion
Half-Life: 40 days
Excretion: Feces >90%; urine <1%
Cautions
Caution in bleeding diathesis, thrombocytopenia, therapeutic anticoagulation
Systemic exposure was increased in patients with moderate hepatic impairment (see Dosage Modifications)
Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception;
Therapy can interfere with estradiol measurement by immunoassay, resulting in falsely elevated estradiol levels;
Injection site related events including sciatica, neuralgia, neuropathic pain, and peripheral neuropathy reported; caution should be taken while administering therapy at dorsogluteal injection site due to proximity of underlying sciatic nerve.
- Related articles
- Related Qustion
- Fulvestrant-Palbociclib vs Letrozole-Palbociclib Mar 4, 2024
Fulvestrant is a selective estrogen receptor (ER)-downregulating antiestrogen that blocks ER transcriptional activity and is approved for ER-positive (+) breast cancer.
- Fulvestrant: A Anti-Estrogen Therapy with Unique Pharmacokinetics and Low Toxicity Feb 7, 2024
Fulvestrant offers stable, safe anti-estrogen therapy with minimal toxicity, requiring no dose adjustment for certain renal or hepatic conditions, but caution in specific populations.
- Fulvestrant: a major breakthrough in breast cancer treatment Aug 31, 2023
Fulvestrant, an anti-estrogenic breast cancer treatment, was approved by the US FDA in September 2002 and approved by the European Union in April 2004 for marketing in Europe.
Tianeptine sodium salt is a salt of Tianeptine, chemically described as [3-chloro-6-methyl-5,5-dioxo-6,11-dihydro-(c,f)-dibenzo-(1,2-thiazepine)-11-yl) amino]-7 heptanoic acid, sodium salt[2]. Tianeptine was discovered and patented by The F....
Oct 9,2019Flavors and fragrancesPreparation; Synthetic applications;Ethyl 4-oxocyclohexanecarboxylate....
Oct 9,2019Drug IntermediateFulvestrant
129453-61-8You may like
- Fulvestrant
-

- $30.00 / 1kg
- 2024-04-26
- CAS:129453-61-8
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 2000kg
- Fulvestrant
-

- $0.00 / 1KG
- 2024-03-16
- CAS:129453-61-8
- Min. Order: 100g
- Purity: 98%+
- Supply Ability: 100kg
- Fulvestrant
-

- $0.00 / 25KG
- 2024-03-12
- CAS:129453-61-8
- Min. Order: 1KG
- Purity: 98% HPLC
- Supply Ability: 500kgs