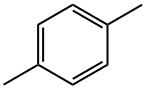
P-xylene
Nov 15,2021
General description
P-xylene appears as a colorless watery liquid with a sweet odor. Less dense than water. Insoluble in water. Irritating vapor. Freezing point is 56°F( 4-Xylene (p-xylene) is a colorless liquid .P-xylene is a xylene with methyl groups at positions 1 and 4. the molecular formula of p-Xylene Commercial or mixed xyleneusually contains about 40-65% m-xylene and up to 20% each of o-xylene and p-xylene and ethylbenzene.
Application and pharmacokinetics
The chemical industry has become more and more widely used, and relevant science and technology have matured. As an important raw material for polyester industry, para-xylene has attracted more and more attention from relevant scholars and enterprises. It is gradually used in various fields, such as the preparation of fibers, resins, films and other materials, and is an essential raw material for synthetic fibers and plastics. Especially in today's era when new technologies are gradually becoming popular, the importance of para-xylene production equipment in its production process is even more prominent.
1.Ibuprofen was prepared from an inactive and inexpensive p-xylene by 3-step flow functionalizations through chemoselective metalations of benzyl positions in sequence using an in-situ generated LICKOR-type superbase. The flow approach in the microreactor facilitated to comprehensively explore over 100 conditions in the first-step reaction by varying concentrations, temperatures, solvents, and equivalents of reagents, enabling to find the optimal condition with 95% yield by significantly suppressing the formation of byproducts, followed by the second C–H metalation step in 95% yield. Moreover, gram-scale synthesis of ibuprofen in the final step was achieved by biphasic flow reaction of solution-phase intermediate with CO2, isolating 2.3 g for 10 min of operation time.[1]
Figure2 Flow approach for the three-step synthesis of ibuprofen from p-xylene through sequential and chemoselective C–H functionalizations
2.it has been reported that p-xylene is present in the urine of PCa patients and has also been indicated as good cancer biomarker. p-xylene is an isomer of xylene, belonging to the aromatic hydrocarbon family, and the aroma of this compound may also be one of those identified in PCa patients by trained dogs. Xylene is lipophilic and is therefore rapidly absorbed via all exposure routes, being evenly distributed between blood and tissues. In humans, around 95% of absorbed xylene is rapidly metabolized, and the metabolites are excreted in urine.The concentration of xylene augments with physical exercise, such as cycling, consistent with the increase in p-xylene levels after prostate stimulation in our patients with PCa. Furthermore, p-xylene was found to be correlated with PSA values and the Gleason score. We observed that the Gleason score was positively correlated with phenol, 2-butanone, and 3-methylphenol in the samples obtained after prostate massage, but the only compound correlated with both Gleason score and PSA values was p-xylene, supporting its potential value as biomarker of PCa.[2]
3.Paraxylene is a very important bulk chemical in the petrochemical industry. It is mainly used to produce terephthalic acid and then to produce polyester, which is an important intermediate for the production of synthetic resins, synthetic fibers and other chemical products. The production of biomass-based para-xylene is of great significance in both academic and industrial fields.
Synthesis
As an important basic chemical raw material, the production process of p-xylene (PX) generally uses naphtha as a material to generate mixed xylenes. In this process, catalytic reforming is generally used, and then PX is separated by adsorption separation technology. The process is selected to be carried out on a multi-stage cryogenic crystallization separation or a molecular sieve simulated moving bed. Through optimization of the process route and integration with toluene methylation technology, the production of P-xylene with naphtha as a raw material increases product selectivity by 30% and minimizes the yield of benzene:
The BP two-stage heavy pulping recovery P-xylene crystallization process technology is selected. The process flow is as follows: after the bottom oil of the depentanization tower from the continuous reforming unit is separated by the reformed oil separation tower, the light reformed oil at the top of the tower enters the aromatic extraction unit, and the heavy reformed oil at the bottom of the tower enters the xylene stripping tower , The other part and the mixed xylene from the disproportionation unit and the xylene isomerization unit enter the xylene recovery tower from different locations. The benzene-rich fraction at the top of the xylene recovery tower and the heavy fraction at the bottom of the tower enter the disproportionation unit, and the xylene is recovered. After a series of heat exchange, the mixed xylene extracted from the side line of the tower enters the crystallization unit raw material tank. The mixed xylene raw material enters the crystallization tank after cooling down. After cooling and crystallization, the crystallization tank bottom slurry is sent to the crystallization tank through the bottom pump. In the decanter centrifuge, after centrifugal separation, the mother liquor enters the isomerization unit as the raw material, and the wet filter cake enters the heavy pulping tank for heavy pulping purification. After the heavy pulping tank bottom slurry is separated by the pusher centrifuge, it is separated After the P-xylene crystal is melted in the product melting tank, it is sent to the product tank outside the boundary as a qualified P-xylene product, and the waste filtrate is returned to the front end for recycling.[3,4]
A method for lignin to directly prepare p-xylene through catalytic conversion, using a suitable metal oxide modified HZSM-5 catalyst, and co-catalyzed pyrolysis of lignin and methanol to produce aromatics mainly containing p-xylene. The monomer compound is accompanied by the alkylation reaction of light aromatic hydrocarbons (such as benzene, toluene) and the isomerization reaction between xylene isomers.
Toxicological and Safety
Xylenes are released into the atmosphere as fugitive emissions from industrial sources, from auto exhaust, and through volatilization from their use as solvents. Acute (short- term) inhalation exposure to mixed xylenes in humans results in irritation of the eyes, nose, and throat, gastrointestinal effects, eye irritation, and neurological effects. Chronic (long-term) inhalation exposure of humans to mixed xylenes results primarily in central nervous system (CNS) effects, such as headache, dizziness, fatigue, tremors, and incoordination; respiratory, cardiovascular, and kidney effects have also been reported. EPA has classified mixed xylenes as a Group D, not classifiable as to human carcinogenicity.
HUMAN EXPOSURE AND TOXICITY: Three women exposed to p-xylene at 100 ppm for 1 to 7.5 hours/day, for 5 days, showed no effects on electroencephalograms, evoked potentials, or cognitive performance, but frequently reported headache and dizziness as a result of exposure. In contrast, four men exposed at concentrations of up to 150 ppm p-xylene under the same exposure conditions reported no increase in headaches or dizziness. Slight impairment of vestibular and visual function and reaction time was noted at exposure levels from 200 to 300 ppm. There was adaption to the impairment over five successive daily exposures.
In a study of levels of noradrenaline and dopamine in the forebrain and hypothalamus, rats (six males/group) were exposed to 0 or 2000 ppm p-xylene 6 hr/day for 3 days. The animals were killed 16-18 hr after the last exposure. In exposed animals there was a significant increase in catecholamine levels and turnover in various parts of the hypothalamus. There was no effect on dopamine levels or turnover in the forebrain. Histological damage to the outer hair cells of the organ of Corti provided evidence of ototoxicity in rats exposed by oral gavage to p-xylene, but not m- or o-xylene, at a dose of 900 mg/kg/day, 5 days/week for 2 weeks. The losses of hair cells occurred in the area of the cochlea responsive to medium frequencies (10-25 kHz). [5]
References
1."From p-Xylene to Ibuprofen in Flow: 3-Step Synthesis via Unified Sequence of Chemoselective C–H Metalations,"
2.Jiménez-Pacheco A., Salinero-Bachiller M. & Iribar M. C. et al., "Furan and p-xylene as candidate biomarkers for prostate cancer," Urologic Oncology: Seminars and Original Investigations, Vol.36, No.5(2018), pp.221-243.
3.Para-xylene (P X) production technology advances
4.Study on optimization of process conditions for isomerization reaction of p-xylene production unit
5.Jia Qifang. Preparation of p-xylene by catalytic pyrolysis of lignin[D]. University of Science and Technology of China, 2018.
- Related articles
- Related Qustion
- How to separate P-XYLENE from xylene? Dec 16, 2024
P-XYLENE is a xylene isomer that is widely used as a feedstock for the manufacture of other industrial chemicals.
Liquid 47% potassium carbonate is typically shipped in drums, tank truck or rail car. It is also available by consignment as a barge load, shipped from the Armand Products’ plant in Muscle Shoals, AL. Each form of transportation has its own....
Nov 15,2021Inorganic chemistryN-[(S)-(4-nitrophenoxy)phenoxyphosphinyl]-L-Alanine 2-ethylbutyl ester is the 6th synthesis intermediate of remdesivir. Remdesivir was developed by Gilead of the United States.....
Nov 15,2021Organic Synthesis IntermediateP-XYLENE
106-42-3You may like
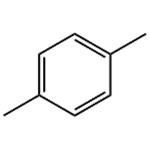
- p-Xylene
-
- $0.00 / 200kg
- 2025-11-19
- CAS:106-42-3
- Min. Order: 20kg
- Purity: 99%
- Supply Ability: 20 tons
- P-XYLENE
-
- $100.00 / 1KG
- 2025-09-25
- CAS:106-42-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- p-Xylene
-

- $150.00 / 1ASSAYS
- 2023-06-26
- CAS:106-42-3
- Min. Order: 10g
- Purity: more than 99.6%
- Supply Ability: 500kg/month