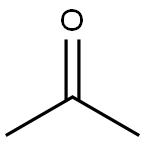
Synthesis and Application of Acetone
May 17,2022
Acetone (ACE one) is an important organic raw material, which is mainly used to produce cellulose acetate film, plastic and coating solvent. Acetone cyanohydrin (ACh) obtained by the reaction of acetone with hydrocyanic acid is the raw material for the preparation of methyl methacrylate vinegar (MMA).
Acetone is also the raw material for preparing bisphenol A, an intermediate of epoxy resin and polycarbonate vinegar. In medicine and pesticide, in addition to being used as raw material of vitamin C, it can also be used as extractant of various microorganisms and hormones, dewaxing solvent for petroleum refining and raw material for manufacturing other synthetic materials.

Synthesis
At present, the production methods of acetone in the world mainly include cumene method, grain fermentation method, isopropanol dehydrogenation method, propylene direct oxidation method (Wacker method), acetic acid by-product method and acetylene water method. Cumene method is the most economical production method of acetone at present, At present, about 93% of acetone in the world is produced by this method.
Process flow diagram of acetone production:[1]
Raw benzene and propylene first generate cumene in the alkylation reactor, and then enter the oxidation reactor to react with oxygen in the air to generate by-products such as cumene hydroperoxide (CHP) and methanol. The intermediate product CHP is concentrated, and its concentration reaches about 82%. Violent decomposition reaction occurs in the decomposition reactor through sulfuric acid catalysis to generate phenol, acetone Cumene and other mixed liquids release a large amount of heat during the reaction, which is carried out by the evaporation of circulating acetone from the top of refined acetone tower.
The mixed liquid is preheated by the feed preheater of the crude acetone tower and enters the crude acetone tower for separation. The light components such as acetone, cumene and water are discharged from the tower top, partially condensed by the condenser of the crude acetone tower and separated in the reflux tank of the crude acetone tower. The vapor phase directly enters the refined acetone tower for further separation, and the liquid phase is used as the reflux of the tower. The refined acetone tower separates acetone from cumene and water to obtain high-purity acetone products from the side line. The top of the tower provides circulating acetone with a mass fraction of less than 2% to the decomposition reactor.
A small amount of dilute alkali liquor is added into the refined acetone tower to remove trace aldehyde impurities in acetone. The discharge from the bottom of the fine acetone tower is cooled by the tower bottom cooler, separated in the separator at the bottom of the fine acetone tower, and the aqueous phase (including phenol) enters the wastewater treatment system after being neutralized by the decomposition liquid; Organic phase is cumene and other components, which are sent to the hydrogenation unit for further treatment.
Application
Acetone is a colorless, volatile, flammable organic solvent. Acetone occurs naturally in plants, trees, forest fires, vehicle exhaust and as a breakdown product of animal fat metabolism. This agent may be normally present in very small quantities in urine and blood; larger amounts may be found in the urine and blood of diabetics. Acetone appears as a clear colorless liquid with a sweetish odor. It occurs naturally in plants, trees, volcanic gases, forest fires, and as a product of the breakdown of body fat. It is present in vehicle exhaust, tobacco smoke, and landfill sites. Industrial processes contribute more acetone to the environment than natural processes.
Toxicological and Safety
Detoxification of aldehydes can proceed by oxidation to readily metabolized acids, by reduction to alcohols, and by reaction with sulfhydryl groups, particularly glutathione. Under conditions that deplete glutathione levels or result in an inhibition of aldehyde dehydrogenase (eg, Antabuse treatment), the acute and chronic effects of aldehyde toxicity might be more fully expressed.
Mitochondria oxidize a variety of aldehydes to acids using oxygen as electron acceptor. Relative rates of oxygen consumption promoted by short-chain aldehydes lie in order: propionaldehyde greater than acetaldehyde greater than formaldehyde.
In vitro, glyoxal has obvious toxic effect on Schwann cells and can eventually lead to apoptosis. Its dose and the degree of damage of Schwann cells are positively correlated with the dose of glyoxal. Therefore, it indicates that the damage of Schwann cells may be related to acetone in diabetic neuropathy.[2]
References
[1]《Process analysis and optimization affecting the quality of acetone products》
[2]Luchengcheng:《Study on the damaging effect of acetone aldehyde on Schwann cells in vitro》,《Chinese medicine》, 2021, No. 01, pp. 134-137.
- Related articles
- Related Qustion
- What is Acetone used for? Oct 13, 2021
The appearance of acetone in scientific thinking may be traced back to 1798, when John Rollo, an English physician, described a material in human breath of an odor of decaying apples. Later this material was identified as acetone, and it wa
Reactions of N-Boc-piperidin-4-one with aromatic aldehydes afforded nitrogen-containing 1,5-diketones in the form of products of intramolecular aldol cyclization, like aza derivatives of tricyclo [7.3.1.02,7]-tridecanone system underlying n....
May 17,2022Pharmaceutical intermediatesDiphenylamine (DPA) is a compound from the third European Union (EU) list of priority pollutants.....
May 19,2022APIAcetone
67-64-1You may like