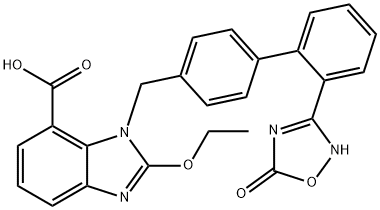
Synthesis of Azilsartan
Sep 16,2021
Azilsartan, the trade name is Azilva, that is 2-ethoxy-1-{[2'-(4,5-dihydro-5-oxo-1,2,4-oxadiazole- 3-yl)biphenyl-4-yl]methyl}-1H-benzo[d]imidazole-7-carboxylic acid. It is an AT1 subtype angiotensin II receptor antagonist (ARB) developed by Takeda, Japan. It was approved to be marketed in Japan on January 18, 2012. It has now been approved for marketing in the United States and the European Union. Azilsartan, as a new generation of dual-function ARBs, not only antagonizes the type 1 receptor of angiotensin II (AT1 receptor), but may also reduce the risk of cardiovascular disease and diabetes through a variety of mechanisms. Clinical trials have shown that Azilsartan has a good curative effect, a low incidence of adverse reactions, and good compliance.
Preparation
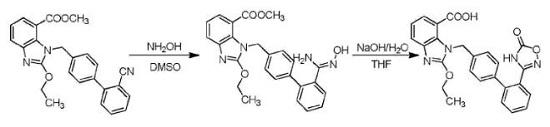
1. Preparation of Azilsartan oxime
Triethylamine (61.8g) was added to the mixed solution of dimethyl sulfoxide (200mL) and hydroxylamine hydrochloride (42.4g), and a large amount of solid precipitated. Filter and wash the filter cake with tetrahydrofuran. The filtrate was concentrated to remove tetrahydrofuran and then added 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1H-benzimidazole-7-carboxylic acid methyl ester 50.0g, reacted at 75℃ for 15 Hour. After the reaction, it was quenched by adding water, and extracted with ethyl acetate. The organic phase was washed with 1mol/L hydrochloric acid, and the aqueous phase was adjusted to pH 10-11 with 1mol/L sodium hydroxide aqueous solution, extracted with ethyl acetate, dried with anhydrous sodium sulfate, filtered, and concentrated to obtain Azilsartan oxime 29.7 g, the yield is 55%.
2.Preparation of Azilsartan
Add 8.8 g of Azilsartan oxime to 20 ml of 10% sodium hydroxide solution, stir at 50°C for 3 hours, and all are dissolved. Control the temperature at 20-30°C, and add a mixed solution of triphosgene (3.0g)/tetrahydrofuran (15ml) dropwise. After the addition is completed, keep the reaction temperature at 20-30°C, continue the reaction for 2-3 hours, after the reaction is completed, adjust the pH to 2-3, precipitate a white solid, and dry it to obtain azilsartan 7.5g with a yield of 83%.
- Related articles
- Related Qustion
- Azilsartan as a Potent Antihypertensive Drug with Possible Pleiotropic Cardiometabolic Effects Jan 15, 2024
Azilsartan, a prodrug and ARB, effectively treats hypertension by inhibiting AT1 receptors. It may provide metabolic benefits and potential organ protection, but further research is required.
- Azilsartan: an angiotensin receptor blocker for treatment of hypertension Jun 26, 2023
Azilsartan is a medication used to treat hypertension and has been shown to exhibit more potent antihypertensive action than other drugs in its class.
Lidocaine hydrochloride was synthesized by L?fgren and Lundquist in 1943, and was clinically introduced in 1948. It remains one of the most widely used local anaesthetics.....
Sep 16,2021API1,2-dimethyloxyethane, also known as glyme, monoglyme, dimethyl glycol, ethylene glycol dimethyl ether, dimethyl cellosolve, and DME, is a colorless, aprotic, and liquid ether that is used as a solvent, especially in batteries.1,2-dimethylo....
Sep 16,2021Organic reagentsAzilsartan
147403-03-0You may like
- Azilsartan
-
- 2025-12-18
- CAS:147403-03-0
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Azilsartan
-

- $0.00 / 1Kg/Bag
- 2025-12-18
- CAS:147403-03-0
- Min. Order: 10g
- Purity: 99.5%min
- Supply Ability: 100kg
- Azilsartan
-

- $0.00 / 1kg
- 2025-12-18
- CAS:147403-03-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20MT