The application of 4′-Methylpropiophenone in organic reactions
Dec 17,2019
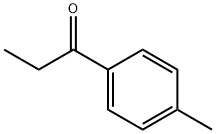
4-Methylpropiophenone is a chemical reagent with an aromatic ketone that is propiophenone bearing a methyl group at C-4. It serves as pharmaceutical and synthesis material intermediate, and it is also used in electrocarboxylation reactions.

4'-methylpropiophenone has been reported to be used in the synthesis of pharmaceutical compounds such as tolperisone and other muscle relaxants. The substance is also a starting material in the synthesis of 4-methylmethcathinone (mephedrone) [1]. Mephedrone has been encountered as a ‘legal high’ and on the illicit drug market masquerading as cocaine (in powder form), MDMA (in tablet form) and as an adulterant. According to the 2016 European Drugs Market Report produced by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), mephedrone has created a specific demand and carved its own distinct market share.
As synthesis mephedrone, the most straightforward route of synthesis for mephedrone is by reacting the suitably substituted bromopropiophenone with methylamine; the resulting product is always racemic [scheme 1]. Therefore, mephedrone is most likely synthesized by bromination of 4-methylpropiophenone (1-(4-methylphenyl)-1-propanone) followed by reaction of the resulting 4-methylbromopropiophenone (1-(4-methylphenyl)-2-bromo-1-propanone) with an excess of methylamine or methylamine hydrochloride and an acid scavenger. The reaction is then quenched with gaseous or aqueous hydrochloride providing the hydrochloride salt that needs to be recrystallised. This is a relatively straightforward option because the starting materials are often commercially available or easily synthesized [2]. The main reason that 4’-Methylpropiophenone was selected as a precursor of mephedrone is because it is a commercial product.
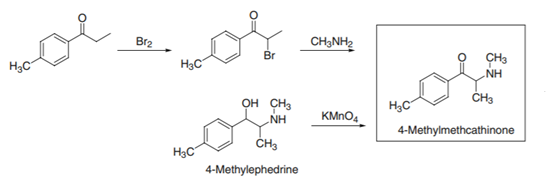
Scheme 1 Synthesis of mephedrone
How do 4'-methylpropiophenone work in electrocarboxylation reactions. The electrochemical activation of aromatic ketones is one of the most important reactions in organic electrochemistry. In recent decades, an electrochemical methodology based on the use of an undivided cell equipped with a sacrificial anode has been a very convenient and cheap way to generate carboxylic acids via the coupling of CO2 and aromatic ketones.
- Related articles
- Related Qustion
- 4'-Methylpropiophenone: A Key Intermediate in Organic Synthesis – Properties, Applications, and Safe Handling Sep 24, 2024
4'-Methylpropiophenone plays a critical role in a wide range of industrial and pharmaceutical applications due to its versatile properties and reactivity.
- 4'-Methylpropiophenone: Applications, synthesis and FTIR May 26, 2023
4-Methylpropiophenone is a chemical reagent with an aromatic ketone that is propiophenone bearing a methyl group at C-4. It serves as pharmaceutical and synthesis material intermediate.
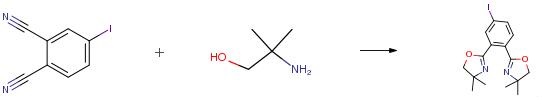
2-Amino-2-methyl-1-propanol is an important organic intermediate (building block) to synthetize substituted amino products.....
Dec 17,2019Organic reagentsAn international team co-led by an Oregon State University chemistry researcher has uncovered a better way to scrub carbon dioxide from smokestack emissions, which could be a key to mitigating global climate change.....
Dec 17,2019API4'-Methylpropiophenone
5337-93-9You may like
4'-Methylpropiophenone manufacturers
- 4'-Methylpropiophenone
-

- $10.00 / 1ASSAYS
- 2025-12-04
- CAS:5337-93-9
- Min. Order: 1ASSAYS
- Purity: 99%
- Supply Ability: 10 ton
- 4-methylpropiophenone
-

- $10.00 / 1ASSAYS
- 2025-12-04
- CAS:5337-93-9
- Min. Order: 1ASSAYS
- Purity: 99%
- Supply Ability: 10 ton
- 1-(4-methylphenyl)-propan-1-one
-

- $10.00 / 1ASSAYS
- 2025-12-04
- CAS:5337-93-9
- Min. Order: 1ASSAYS
- Purity: 99%
- Supply Ability: 10 ton