The applications and synthesize of 2-2-bis-diphenylphosphino-1-1-binaphthyl
Aug 19,2019
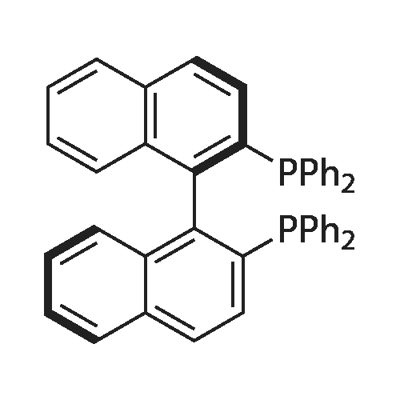
BINAP (2,2'-bis(diphenylphosphino)-1,1'-binaphthyl) is an organophosphorus compound. This chiral ligand is widely used in asymmetric synthesis. It consists of a pair of 2-diphenylphosphinonaphthyl groups linked at the 1 and 1´ positions. This C2-symmetric framework lacks a stereogenic atom, but has axial chirality due to restricted rotation (atropisomerism). The barrier to racemization is high due to steric hindrance, which limits rotation about the bond linking the naphthyl rings. The dihedral angle is approximately 90˚.
The following example is about its application on the synthesis of benzoxazine derivatives, where BINAP is a chiral ligand [1]
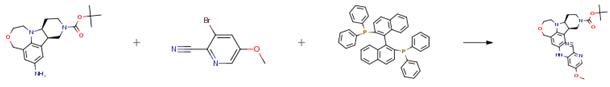
The title compound was prepared using tert-butyl (7bR,11aS)-6-amino-1,2,7b,10, 11,11a-hexahydro-4H-[1,4]oxazepino[6,5,4-hi]pyrido[4,3-b]indole-9(8H)-carboxylate from Example 56, Part B (130 mg, 0.377 mmol), 3-bromo-2-cyano-5- methoxypyridine (67 mg, 0.34 mmol), CsCO3 (256 mg, 0.787 mmol) in anhydrous toluene (10 mL), tris(dibenzylideneacetone)dipalladium (3.4 mg, 3.7 μmol), and 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl (7.0 mg, 11 μmol) to provide the corresponding tert-butyl (7bR,11aS)-6-(2-cyano-5-methoxy-3-pyridinyl)-amino -1,2,7b,10,11,11a-hexahydro-4H-[1,4]oxazepino[6,5,4-hi]pyrido[4,3-b]indole-9(8H)-carboxylate (146 mg) in 90 percent yield.
The following example is about its application on the synthesis of diazabicyclic agents, where BINAP is a chiral catalyst. [3]
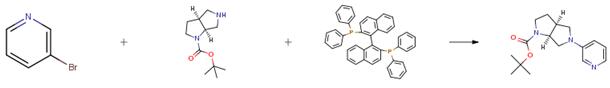
The starting material (0.71 g, 3.30 mmol) in toluene (33 mL) was treated with tris(dibenzylideneacetone)dipalladium(0) (61 mg, 0.10 mmol), 2,2'-bis(diphenyl phosphino)-1,1'-binaphthyl (BINAP) (83 mg, 0.10 mmol), 3-bromopyridine (0.58 g, 3.70 mmol), and sodium tert-butoxide (0.54 g, 5.60 mmol). After heating at 80° C. for 16 hours, the mixture was poured into diethyl ether (100 mL), washed with brine (100 mL), dried (MgSO4) and concentrated under reduced pressure. The residue was purified by chromatography (SiO2, 5 percent MeOH/CH2Cl2) to provide the product as a yellow oil (0.87 g, 91percent yield).
The following example is about its application on the synthesis of substituted azepino[4,5b] indoline derivatives, where BINAP is a chiral ligand [4]
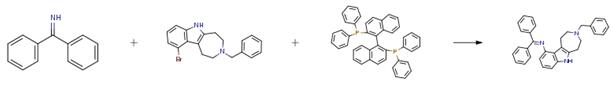
A mixture of 3-benzyl-10-bromo-1,2,3,4,5,6-hexahydroazepino[4,5-b]indole (0.71 g, 2.00 mmol), benzophenone imine (0.44 g, 0.40 mL, 2.40 mmol), tris(dibenzyl ideneacetone)dipalladium(0) (0.037 g, 0.040 mmol), (S)-(-)-2,2'-bis(diphenyl phosphino)-1,1'-binaphthyl (0.075 g, 0.120 mmol), and sodium tert-butoxide (0.269 g, 2.80 mmol) in toluene (20.0 mL) was refluxed for 16 h. After cooling to room temperature, water and ethyl acetate were added, and the phases separated. The aqueous layer was extracted with ethyl acetate. The combined ethyl acetate solution was dried (MgSO4) and filtered. The filtrate was concentrated in vacuo to dryness and the residue was subjected to column chromatography (silica gel, 30 percent EtOAc/hexane, 1 percent Et3N) to afford a yellow fluffy solid as the product (0.855 g, 94 percent): mp > 71° C.
References
1. Roche Palo Alto LLC. Benzoxazine derivatives and uses thereof US2003/232825[P], 2003, A1, Page 31.
2. Robichaud AJ, Fevig JM, Mitchell IS, Lee T, Chen W, Cacciola J. Substituted pyridoindoles as serotonin agonists and antagonists. US2004/186094[P], 2004, A1.
3. Schrimpf MR, Tietje KR, Toupence RB, Ji J, Basha A, Bunnelle WH, Daanen JF, Pace JM, Sippy KB. Diazabicyclic central nervous system active agents. US2002/19388[P], 2002, A1.
4. Frank KE, Acker BA, Ennis MD, Fisher JF, Fu J, Jacobsen EJ, McWhorter JR, William W, Morris JK, Rogier DJ. Substituted azepino[4,5b] indoline derivatives. US2002/77318[P], 2002, A1
- Related articles
- Related Qustion
- (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl: properties and applications in organic synthesis Nov 23, 2023
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl is a crucial and versatile chiral ligand in asymmetric catalysis, enabling highly selective organic synthesis.
Voglibose (INN and USAN, trade name Voglib, marketed by Mascot Health Series) is an alpha-glucosidase inhibitor used for lowering post-prandial blood glucose levels in people with diabetes mellitus. Voglibose delays the absorption of glucose thereby reducing the risk of macrovascular complications. Voglibose is a research product of Takeda Pharmaceutical Company, Japan's largest pharmaceutical company. Voglibose was first launched in 1994, under the trade name BASEN, to improve postprandial hyperglycemia in diabetes mellitus.....
Aug 19,2019APIIsoginkgetin inhibits splicing both in vivo and in vitro at similar micromolar concentrations. It appears to do so by preventing stable recruitment of the U4/U5/U6 tri-small nuclear ribonucleoprotein, resulting in accumulation of the prespliceosomal A complex. Like two other recently reported general pre-mRNA splicing inhibitors, isoginkgetin has been previously described as an anti-tumor agent.....
Aug 19,2019Natural ProductsYou may like
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl manufacturers
- (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl
-
- $10.00 / 1KG
- 2024-04-26
- CAS:76189-55-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl
-
- $230.00 / 5g
- 2024-02-23
- CAS:76189-55-4
- Min. Order: 1g
- Purity: 0.98
- Supply Ability: 25kg
- (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl
-

- $0.00 / 25KG
- 2023-07-28
- CAS:76189-55-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month