What is Bis(pinacolato)diboron?
Feb 25,2020
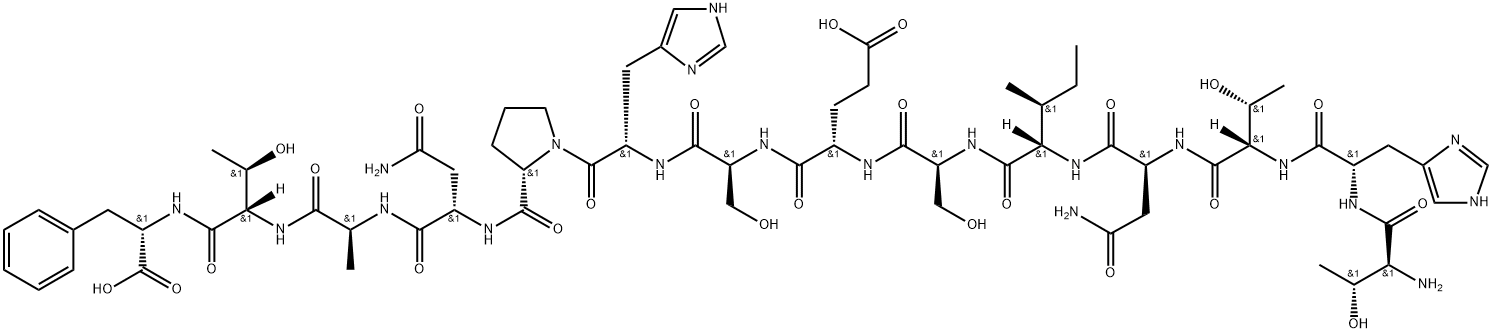
Bis(pinacolato)diboron(L-Threonyl-L-histidyl-L-threonyl-L-asparaginyl-L-isoleucyl-L-seryl-L-alpha-glutamyl-L-seryl-L-histidyl-L-prolyl-L-asparaginyl-L-alanyl-L-threonyl-L-phenylalanine,C66H98N20O24) appears as powder or platelets. Bis(pinacolato)diboron is soluble in tetrahydrofuran, dichloromethane, toluene, hexane and heptane and insoluble in water.
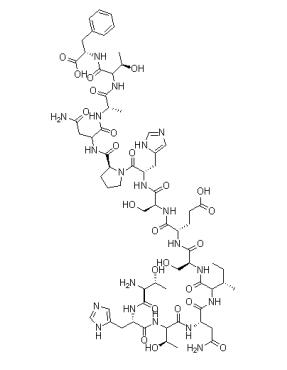
Fig 1. Chemical structure formula of Bis(pinacolato)diboron
Preparation:The specific steps of a Bis (pinacolato) diboron synthesis method are as follows:
1) Triamino substitution: Add the hydrocarbon solution of dimethylamine, the main raw material, to the reactor, and add the hydrocarbon solution of boron trichloride dropwise at -20℃to 0℃. After the drop is completed, the temperature of the system is raised to 5~25℃. Until the end of the NMR detection reaction; after the reaction is completed, a hydrocarbon solvent is added to the system, stirred, and centrifuged, and the obtained filtrate is a hydrocarbon solution of tris (dimethylamino) borane, which is to be used in the next step; The amount of the solvent is 2 to 14 mL of dimethylamine per gram of the main raw material, and the amount of the hydrocarbon solvent to dissolve boron trichloride is 1 to 7 mL of dimethylamine, which is the main raw material. The molar ratio is 1.0: 0.17~0.3 eq. The amount of the hydrocarbon solvent added after the reaction is 1 to 5 mL of dimethylamine as the main raw material;
2) Diamino substitution: Add the hydrocarbon solution of tris (dimethylamino) borane obtained in step (1) to the reactor, and add the hydrocarbon solution of boron trichloride dropwise at -20 to 0℃. After the reaction, the system was heated to 5~25℃ and kept warm until the end of the NMR detection reaction. After the reaction was completed, the system was passed through diatomaceous earth. The resulting filtrate was a bis (dimethylamino) chloroborane hydrocarbon solution, which was used for the next step. The amount of boron chloride hydrocarbon solvent is 1 to 9 mL per gram of the main raw material bis (dimethylamino) chloroborane, and the molar ratio of the main raw material bis (dimethylamino) chloroborane and boron trichloride is 1.0. : 0.5~1.0eq; the weight ratio of the main raw material bis (dimethylamino) chloroborane to diatomite is 1: 0.1~1;
3) Coupling: Add a hydrocarbon solvent and sodium metal to the reactor, stir and reflux until the sodium metal completely melts, and dropwise add the bis (dimethylamino) chloroborane obtained in step (2) at 85-105℃. After the reaction is completed, the temperature is kept constant until the NMR detection reaction is completed. After the reaction is completed, the system is cooled to 20~40℃, filtered, and the filtrate is concentrated to a non-fraction to obtain tetra (dimethylamino) -diborane. The amount of the similar solvent is 1 to 10 mL of bis (dimethylamino) chloroborane per gram of the main raw material, and the molar ratio of the main raw material bis (dimethylamino) chloroborane to the sodium metal is 1.0: 1.0 to 3.0eq;
4) Esterification: A hydrocarbon solvent and the tetrakis (dimethylamino) -diborane obtained in the above step (3) are sequentially added to the reactor, and pinacol is added dropwise at 10-30℃. After the drop is completed, the system is heated up. Keep the temperature to 85~105℃, keep the temperature unchanged until the end of the NMR detection reaction. After the reaction is completed, the system is cooled down to 15~25℃. Water and a hydrocarbon solvent are added for extraction. The liquid phase is separated. The obtained organic phase is washed with saturated brine, dried, and filtered. Concentrate to no fractions to obtain pinacol borate; the amount of hydrocarbon solvents to be used is 1 to 10 mL of tetrakis (dimethylamino) ‑diborane per gram of main raw material, and tetrakis (dimethylamino) ‑diboron as main raw material. The molar ratio of alkane to pinacol is 1.0: 1.5 to 3.0 eq. The amount of water used is 1 to 10 ml of tetrakis (dimethylamino) ‑diborane per gram of main raw material, and the amount of extracted hydrocarbon solvents is gram per gram of main raw material. The raw material tetrakis (dimethylamino) -diborane is 1~10 mL, and the amount of saturated saline solution is 1~10mL of tetrakis (dimethylamino) -diborane per gram of the main raw material)[1].
Application:In the Suzuki reaction, Bis (pinacolato) diboron has the advantages of high reaction selectivity, mild conditions, and high yield. For some compounds that are unstable or need to improve the selectivity of the reaction, Bis (pinacolato) diboron and aryl halide are used. React is a good choice.
Bis (pinacolato) diboron application examples are as follows:
1. Preparation of an alkenyl borate ester, including a substrate copper salt catalyst, a ligand, a base and a biboronic acid pinacol ester, in a reaction system of the substrate and the diboronic acid pinacol The ester undergoes boron dehydrogenation. This method realizes simple dehydroboronation of olefins under milder conditions (80-100℃), and uses this method to synthesize a bioactive molecule with an estrone structure, which can be modified in the next step to be converted into useful Drug structure.
2.Preparation of 3,4-dimethoxy-5-methylphenylboronic acid pinacol. 3,4-dimethoxy-5-methylphenylboronic acid pinacol ester as a novel borate synthetic building block can provide a new structure for the development of new drugs, and will contain methyl methoxy substituted benzene ring It is used in the synthesis of natural products. The method is realized by the following steps: 1) dissolving an iridium reagent, a ligand, and Bis (pinacolato) diboron in a solvent and stirring until dark green to obtain an intermediate product; 2) adding 2,3-dimethylformate to the intermediate product Oxytoluene, heated to reflux for 6-9 hours to obtain the crude product of the target; 3) The crude product of 3,4-dimethoxy-5-methylphenylboronic acid pinacol ester was concentrated and spin-dried 3,4-Dimethoxy-5-methylphenylboronic acid pinacol ester was separated by a column. In the present invention, 3,4-dimethoxy-5-methylphenylboronic acid pinacol ester is synthesized by using commercially available 2,3-dimethoxytoluene and pinacol diborate as starting materials to prepare The method is simple, the separation is convenient, the yield is high, the selectivity is good, and the reagents used are common reagents, which is cheap and easy to obtain[2-3].
References
[1] Method for synthesizing biboronic acid pinacol ester. CN201210054133.8.
[2] Method for synthesizing copper-catalyzed alkenyl borate. CN201811236690.5.
[3] Preparation method of 3,4-dimethoxy-5-methylphenylboronic acid pinacol ester. CN201611185633.X.
Bis(pinacolato)diboron(L-Threonyl-L-histidyl-L-threonyl-L-asparaginyl-L-isoleucyl-L-seryl-L-alpha-glutamyl-L-seryl-L-histidyl-L-prolyl-L-asparaginyl-L-alanyl-L-threonyl-L-phenylalanine,C66H98N20O24) appears as powder or platelets. Bis(pinacolato)diboron is soluble in tetrahydrofuran, dichloromethane, toluene, hexane and heptane and insoluble in water.
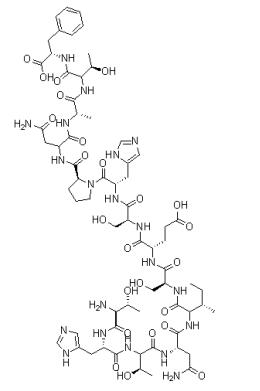
Fig 1. Chemical structure formula of Bis(pinacolato)diboron
Preparation:The specific steps of a Bis (pinacolato) diboron synthesis method are as follows:
1) Triamino substitution: Add the hydrocarbon solution of dimethylamine, the main raw material, to the reactor, and add the hydrocarbon solution of boron trichloride dropwise at -20℃to 0℃. After the drop is completed, the temperature of the system is raised to 5~25℃. Until the end of the NMR detection reaction; after the reaction is completed, a hydrocarbon solvent is added to the system, stirred, and centrifuged, and the obtained filtrate is a hydrocarbon solution of tris (dimethylamino) borane, which is to be used in the next step; The amount of the solvent is 2 to 14 mL of dimethylamine per gram of the main raw material, and the amount of the hydrocarbon solvent to dissolve boron trichloride is 1 to 7 mL of dimethylamine, which is the main raw material. The molar ratio is 1.0: 0.17~0.3 eq. The amount of the hydrocarbon solvent added after the reaction is 1 to 5 mL of dimethylamine as the main raw material;
2) Diamino substitution: Add the hydrocarbon solution of tris (dimethylamino) borane obtained in step (1) to the reactor, and add the hydrocarbon solution of boron trichloride dropwise at -20 to 0℃. After the reaction, the system was heated to 5~25℃ and kept warm until the end of the NMR detection reaction. After the reaction was completed, the system was passed through diatomaceous earth. The resulting filtrate was a bis (dimethylamino) chloroborane hydrocarbon solution, which was used for the next step. The amount of boron chloride hydrocarbon solvent is 1 to 9 mL per gram of the main raw material bis (dimethylamino) chloroborane, and the molar ratio of the main raw material bis (dimethylamino) chloroborane and boron trichloride is 1.0. : 0.5~1.0eq; the weight ratio of the main raw material bis (dimethylamino) chloroborane to diatomite is 1: 0.1~1;
3) Coupling: Add a hydrocarbon solvent and sodium metal to the reactor, stir and reflux until the sodium metal completely melts, and dropwise add the bis (dimethylamino) chloroborane obtained in step (2) at 85-105℃. After the reaction is completed, the temperature is kept constant until the NMR detection reaction is completed. After the reaction is completed, the system is cooled to 20~40℃, filtered, and the filtrate is concentrated to a non-fraction to obtain tetra (dimethylamino) -diborane. The amount of the similar solvent is 1 to 10 mL of bis (dimethylamino) chloroborane per gram of the main raw material, and the molar ratio of the main raw material bis (dimethylamino) chloroborane to the sodium metal is 1.0: 1.0 to 3.0eq;
4) Esterification: A hydrocarbon solvent and the tetrakis (dimethylamino) -diborane obtained in the above step (3) are sequentially added to the reactor, and pinacol is added dropwise at 10-30℃. After the drop is completed, the system is heated up. Keep the temperature to 85~105℃, keep the temperature unchanged until the end of the NMR detection reaction. After the reaction is completed, the system is cooled down to 15~25℃. Water and a hydrocarbon solvent are added for extraction. The liquid phase is separated. The obtained organic phase is washed with saturated brine, dried, and filtered. Concentrate to no fractions to obtain pinacol borate; the amount of hydrocarbon solvents to be used is 1 to 10 mL of tetrakis (dimethylamino) ‑diborane per gram of main raw material, and tetrakis (dimethylamino) ‑diboron as main raw material. The molar ratio of alkane to pinacol is 1.0: 1.5 to 3.0 eq. The amount of water used is 1 to 10 ml of tetrakis (dimethylamino) ‑diborane per gram of main raw material, and the amount of extracted hydrocarbon solvents is gram per gram of main raw material. The raw material tetrakis (dimethylamino) -diborane is 1~10 mL, and the amount of saturated saline solution is 1~10mL of tetrakis (dimethylamino) -diborane per gram of the main raw material)[1].
Application:In the Suzuki reaction, Bis (pinacolato) diboron has the advantages of high reaction selectivity, mild conditions, and high yield. For some compounds that are unstable or need to improve the selectivity of the reaction, Bis (pinacolato) diboron and aryl halide are used. React is a good choice.
Bis (pinacolato) diboron application examples are as follows:
1. Preparation of an alkenyl borate ester, including a substrate copper salt catalyst, a ligand, a base and a biboronic acid pinacol ester, in a reaction system of the substrate and the diboronic acid pinacol The ester undergoes boron dehydrogenation. This method realizes simple dehydroboronation of olefins under milder conditions (80-100℃), and uses this method to synthesize a bioactive molecule with an estrone structure, which can be modified in the next step to be converted into useful Drug structure.
2.Preparation of 3,4-dimethoxy-5-methylphenylboronic acid pinacol. 3,4-dimethoxy-5-methylphenylboronic acid pinacol ester as a novel borate synthetic building block can provide a new structure for the development of new drugs, and will contain methyl methoxy substituted benzene ring It is used in the synthesis of natural products. The method is realized by the following steps: 1) dissolving an iridium reagent, a ligand, and Bis (pinacolato) diboron in a solvent and stirring until dark green to obtain an intermediate product; 2) adding 2,3-dimethylformate to the intermediate product Oxytoluene, heated to reflux for 6-9 hours to obtain the crude product of the target; 3) The crude product of 3,4-dimethoxy-5-methylphenylboronic acid pinacol ester was concentrated and spin-dried 3,4-Dimethoxy-5-methylphenylboronic acid pinacol ester was separated by a column. In the present invention, 3,4-dimethoxy-5-methylphenylboronic acid pinacol ester is synthesized by using commercially available 2,3-dimethoxytoluene and pinacol diborate as starting materials to prepare The method is simple, the separation is convenient, the yield is high, the selectivity is good, and the reagents used are common reagents, which is cheap and easy to obtain[2-3].
References
[1] Method for synthesizing biboronic acid pinacol ester. CN201210054133.8.
[2] Method for synthesizing copper-catalyzed alkenyl borate. CN201811236690.5.
[3] Preparation method of 3,4-dimethoxy-5-methylphenylboronic acid pinacol ester. CN201611185633.X.
- Related articles
- Related Qustion
- Bis(pinacolato)diboron: Synthesis and applications May 22, 2023
Bis(pinacolato)diboron is an important organic intermediate. In Suzuki reaction, it has the advantages of high reaction selectivity, mild conditions, and high yield.
Tegoprazan was approved by the Ministry of Food and Drug Safety (MFDS) for marketing in July 2018 for the treatment of gastroesophageal reflux disease and erosive esophagitis. Tegoprazan was originally developed by Pfizer.....
Feb 24,2020DrugsTrametinib, a reversible inhibitor of mitogen-activated extracellular signal-regulated kinase 1 (MEK1) and MEK2, can influence the MAPK pathway and inhibit the cellular proliferation by activation of MEK1 and MEK2 kinases.....
Feb 25,2020InhibitorsTegoprazan was approved by the Ministry of Food and Drug Safety (MFDS) for marketing in July 2018 for the treatment of gastroesophageal reflux disease and erosive esophagitis. Tegoprazan was originally developed by Pfizer.....
Feb 24,2020DrugsTrametinib, a reversible inhibitor of mitogen-activated extracellular signal-regulated kinase 1 (MEK1) and MEK2, can influence the MAPK pathway and inhibit the cellular proliferation by activation of MEK1 and MEK2 kinases.....
Feb 25,2020InhibitorsYou may like
- 4,4,4',4',5,5,5',5'-Octamethyl-2,2'-bi-1,3,2-dioxaborolane
-

- $1.00 / 1KG
- 2020-01-10
- CAS:78183-34-3
- Min. Order: 1KG
- Purity: 98% HPLC
- Supply Ability: 10 tons/month