What is Linagliptin?
Feb 10,2020
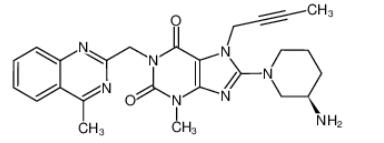
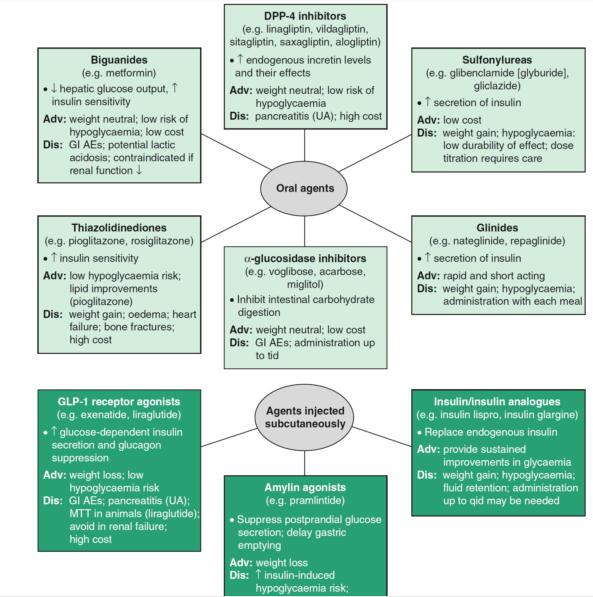
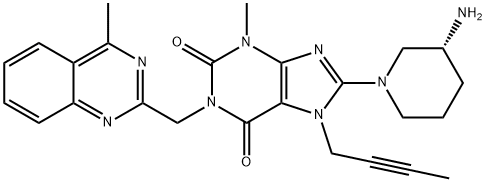
Linagliptin (TrajentaR, TradjentaTM, TrazentaTM, TrayentaTM) is an oral, highly selective inhibitor of dipeptidyl peptidase-4 and is the first agent of its class to be eliminated predominantly via a nonrenal route. Linagliptin is indicated for once daily use for the treatment of adults with type 2 diabetes mellitus.[1]
Linagliptin acts to lower blood glucose levels by inhibiting the enzyme DPP-4, thereby preventing the degradation of the incretin hormones (glucagon-like peptide-1 [GLP-1] and glucose-dependent insulinotropic peptide) and attenuating postprandial glucose excursions. By selectively targeting DPP-4, linagliptin potentially causes a more physiologically based control of glucose-dependent postprandial glucose excursions and of fasting blood glucose, both of which are mediated by effects of glucose on insulin and glucagon secretion. An advantage of linagliptin is that since incretin-stimulated release of insulin is glucose dependent, linagliptin is associated with a low incidence of hypoglycaemia. Moreover, DPP-4 inhibitors have a low potential for drug-drug interactions (with the exception of saxagliptin, which is metabolized by cytochrome P450 [CYP] 3A4/5), are generally well tolerated and have minimal or neutral effects on bodyweight. [2]
Linagliptin shows modest oral bioavailability, and it is rapidly absorbed. The maximum plasma concentration at steady state is reached on average 1.5 hours after administration of linagliptin 5 mg, once daily . Linagliptin half-life is 131 hours. No relevant food effects were observed on the absorption profile of linagliptin. Unlike other DPP-4 inhibitors, linagliptin excretion is not performed by the kidneys, but rather through the enterohepatic system. [3]
Linagliptin, as monotherapy or in combination with other oral antihyperglycaemic drugs, was generally well tolerated in clinical trials, having neutral or minimal effects on bodyweight and generally being associated with a very low incidence of hypoglycaemia. In addition, clinical data currently available indicate that linagliptin is an effective and generally well tolerated treatment option for use in patients with type 2 diabetes, including those with renal impairment for whom other antihyperglycaemia agents require dosage adjustment or are not suitable. [4]
In May 2011, linagliptin was approved by the US Food and Drug Administration for the treatment of type 2 diabetes as a monotherapy or together with different commonly prescribed oral antidiabetes drugs - metformin, sulfonylurea, pioglitazone.
References
1.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW: 10-Year follow-up of intensive glucose control in Type 2 Diabetes[J]. N Engl J Med 2008, 359:1577–1589
2.DeFronzo AR: From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes melitus[J]. Diabetes 2009, 58:773–795.
3.Gupta V, Kalra S: Choosing a gliptin[J]. Indian J Endocrinol Metab 2011, 14(4):298–308.
Scheen AJ: A review of gliptins in 2011[J]. Expert Opn Pharmacolther 2012, 13(1):81–99.
- Related articles
- Related Qustion
- Linagliptin: A Potent DPP-4 Inhibitor for Effective Management of Type 2 Diabetes Mellitus Nov 11, 2024
Linagliptin is a potent, selective dipeptidyl peptidase-4 (DPP-4) inhibitor used in the management of type 2 diabetes mellitus (T2DM).
See also
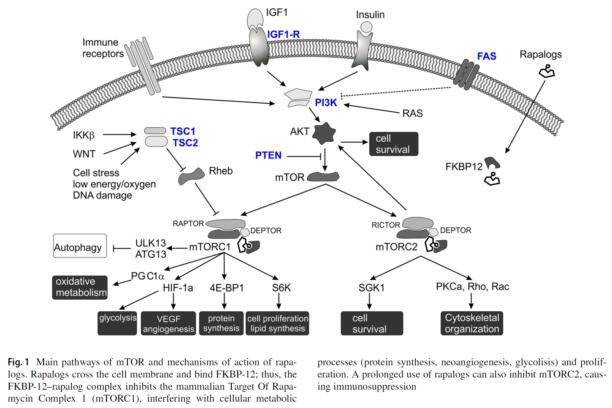
Sirolimus, also known as rapamycin, is a macrolide derived from Streptomyces hygroscopicus. Sirolimus and its analog molecules, the so called ‘rapalogs’, belong to the inhibitors of the mammalian target of rapamycin (mTOR).....
Feb 10,2020InhibitorsIbrutinib is a first-in-class,potent, orally administered covalently-binding inhibitor of BTK.....
Feb 10,2020DrugsLinagliptin
668270-12-0You may like
- Linagliptin
-
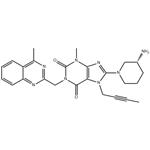
- 2025-12-02
- CAS:668270-12-0
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Linagliptin
-

- $0.00 / 1Kg/Bag
- 2025-12-02
- CAS:668270-12-0
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 50kg
- Linagliptin
-
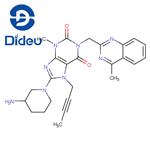
- $0.00 / 25kg
- 2025-12-01
- CAS:668270-12-0
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1000kg